Haryana Board (HBSE) Class 12 Chemistry Question Paper 2023 Answer Key. Haryana Board Class 12th Chemistry Solved Question Paper 2023 PDF Download. HBSE Board Solved Question Paper Class 12 Chemistry 2023. HBSE 12th Question Paper Download 2023. HBSE Class 12 Chemistry Previous Year Question Paper with Answer. HBSE 12th Chemistry Solved Question Paper 2023. HBSE 12th Class Chemistry Solved Question Paper 2023.
HBSE Class 12 Chemistry Question Paper 2023 Answer Key (All Sets – A,B,C,D)
SET–A
Q1.(i) Isotonic solutions are those solutions which have same :
(A) Concentration
(B) Osmotic Pressure
(C) Surface Tension
(D) Viscocity
Ans : (B) Osmotic Pressure
(ii) Standard Electrode Potential for Standard Hydrogen Electrode (SHE) is :
(A) -0.5 V
(B) +1.0 V
(C) 0.0 V
(D) +2.0 V
Ans : (C) 0.0 V
(iii) Identify the order of reaction from the given rate constant K = 1.6 × 10-⁶ L Mol-¹ Sec-¹ :
(A) Zero
(B) First
(C) Second
(D) None of these
Ans : (C) Second
(iv) What is the co-ordination number of Cobalt in the [Co(NH3)5(CO3)]Cl compound ?
(A) 4
(B) 6
(C) 8
(D) 5
Ans : (B) 6
(v) Which one of the following has the highest dipole moment ?
(A) CHCl3
(B) CH3Cl
(C) CH2Cl2
(D) CCl4
Ans : (B) CH3Cl
(vi) In the reaction C6H5OCH3 + HI + 373K → A + B, A and B are :
(A) C6H5I, CH3OH
(B) C6H5OH, CH3I
(C) C6H5CH2OH, CH3I
(D) CH3CH2I, C6H5OH
Ans : (B) C6H5OH, CH3I
(vii) IUPAC name of the compound C6H5CH=CHCHO is …………….
Ans : 3-phenylprop-2-enal
(viii) Ethyl amine is soluble in water but Aniline does not.
Ans : Due to absence of hydrogen bonding in Aniline.
(ix) Which base is present in RNA but not in DNA ?
(A) Thymine
(B) Cytosine
(C) Guanine
(D) Uracil
Ans : (D) Uracil
(x) Order of reaction is …………. when K = 3 × 10-⁴ s-¹.
Ans : First order
(xi) Complete the following equation :
CH3CH2OH + Conc. H2SO4 + 443 K →
Ans : CH2=CH2 + H2O
(xii) Deficiency of Vitamin ‘D’ causes ……………
Ans : Rickets (bone weakness or bone deformity)
(xiii) What are isotonic solutions ?
Ans : Isotonic solutions are those solutions which have same Osmotic Pressure.
(xiv) Write IUPAC name of K3[Fe(CN)6] Compound.
Ans : Potassium hexacyanoferrate (III)
(xv) Why Phenol is more Acidic then Alcohol ?
Ans : Phenol is more acidic than alcohols due to stabilisation of phenoxide ion through resonance.
Q2. Calculate the Molarity of a solution containing 4g NaOH in 200 ml of solution.
Ans : Given mass = 4 g
Molar mass of NaOH = 23 + 16 + 1 = 40 g/mol
No. of moles = Given mass / Molar mass = 4/40 = 0.1 moles
Volume of solution = 200 mL = 200/1000 = 0.2 L
Molarity = No. of moles / Volume of solution (in L) = 0.1/0.2 = 0.5 mol/L
Q3. Define Catalyst and Activation Energy.
Ans : Catalyst – A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. It works by lowering the activation energy required for the reaction to occur, thereby increasing the number of successful collisions between reactant molecules.
Activation Energy – Activation energy is the minimum amount of energy required for a chemical reaction to occur. It is the energy that must be supplied to reactant molecules in order to break the existing bonds and initiate the reaction. The activation energy determines the reaction rate, as a higher activation energy usually leads to a slower reaction rate.
Q4. What is co-ordination number? What will be the co-ordination number of Pt in [PtCl6]-² ?
Ans – Co-ordination number refers to the number of atoms or ligands that are directly bonded to a central atom in a complex. In other words, it represents the number of attachment points around the central atom.
Let x be the oxidation number of Pt in [PtCl6]-²
The Cl atoms have oxidation number of -1.
x + 6(-1) = -2
x – 6 = -2
x = +4
Hence, the oxidation state of platinum in [PtCl6]-² is + 4.
Q5. In the following pairs of halogen compound which would undergo SN² reaction faster ?
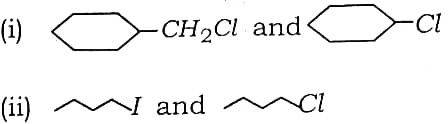
Ans – (i) first compound (–CH2Cl)
(ii) first compound (–I)
Q6. Why transition metals and many of their compounds show paramagnetic behaviour ?
Ans – Paramagnetic arises due to the presence of unpaired electrons. Each such electrons has a magnetic moment associated with it due to its spin angular momentum. Transition metals have in its ground state or ionised state have number of unpaired d-electrons which gives it a paramagnetic behaviour.
Q7. Explain with example Kolbe’s reaction.
Ans – Kolbe’s reaction, also known as Kolbe Schmitt Reaction, is a type of addition reaction named after Hermann Kolbe and Rudolf Schmitt. When phenol is treated with sodium hydroxide, phenoxide ion is generated. The phenoxide ion generated is more reactive than phenol towards electrophilic aromatic substitution reaction. Hence, it undergoes an electrophilic substitution reaction with carbon dioxide, which is a weak electrophile. Ortho-hydroxybenzoic acid (salicylic acid) is formed as the primary product. This reaction is popularly known as Kolbe’s reaction.
Q8. Give reason for the higher boiling point of ethanol in comparison to methoxymethane ?
Ans – Ethanol undergoes intermolecular H-bonding due to the presence of -OH group, resulting in molecule interaction. Extra energy is required to break these hydrogen bonds. On the other hand, methoxymethane does not undergo Hydrogen bonding. Hence, the boiling point of ethanol is higher than that of methoxymethane.
Q9. Write down the Gattermann reaction, and why this reaction preferred over Sandmeyer reaction ?
Ans – The gattermann reaction can be defined as a method of formylation of aromatic ring compounds. The reaction is also known by the names of Gattermann salicylaldehyde synthesis as well as gattermann formylation.
The Gattermann reaction is preferred over the Sandmeyer reaction because it is a milder and less hazardous method for the synthesis of aldehydes from aromatic compounds.
Q10.(i) State Henry’s law and give two applications.
Ans – Henry’s Law states that the solubility of a gas in liquid is directly proportional to the partial pressure of the gas.
Applications : In deep sea diving and In aerated water e.g. soft drinks.
(ii) Why do gases always tend to be less soluble in liquids as the temperature is raised?
Ans – Dissolution of gas is an exothermic process. As the temperature is raised, the equilibrium shifts in reverse direction (Le-Chatelier’s principle). It results in decrease of solubility of gases in liquid.
Q11. How much charge required in Coulomb for the following reductions or oxidations ?
(i) 1 mole of Al³+ to Al
Ans – Required charge = 3 F = 3 × 96500 C = 289500 C
(ii) 1 mole of H2O to O2
Ans – Required charge = 2 F = 2 × 96500 C = 193000 C
(iii) 1 mole of MnO4– to Mn²+
Ans – Required charge = 5 F = 5 × 96500 C = 482500 C
Q12. A first order reaction takes 40 min. for 30% decomposition. Calculate half-life period.
Ans – t = 40 min, [R]° = 100, [R] = 100-30 = 70
For a first order reaction,
t = 2.303/k Log [R]°/[R]
k = 2.303/t Log [R]°/[R]
k = 2.303/40 Log[100/70] = 2.303/40 × Log[1.429] = 2.303/40 × 0.155 = 0.008918 min-¹
Therefore, t½ of the decomposition reaction is
t½ = 0.693/k = 0.693/0.008918 = 77.7 min (approximately)
Q13. What is meant by unidentate, didentate, and ambidentate ligand? Give an example of each.
Ans – Unidentate Ligand – Unidentate ligand refers to a ligand that can form only one coordinate bond with a metal ion. For example, chloride ion (Cl-) is a unidentate ligand because it can form only one coordinate bond with a metal ion.
Didentate Ligand – Didentate ligand refers to a ligand that can form two coordinate bonds with a metal ion. For example, ethylenediamine (en) is a didentate ligand because it can form two coordinate bonds with a metal ion.
Ambidentate Ligand – Ambidentate ligand refers to a ligand that can form coordinate bonds through two different atoms. For example, cyanide ion (CN-) is an ambidentate ligand because it can form a coordinate bond through either the carbon or nitrogen atom.
Q14. Write short notes on :
(a) Freon
Ans – Freon is a brand name for a group of chlorofluorocarbon (CFC) and hydrochlorofluorocarbon (HCFC) compounds. They were widely used as refrigerants and propellants but have been phased out due to their harmful environmental effects. Freons are colourless, odourless, nonflammable, noncorrosive gases or liquids of low toxicity.
(b) D.D.T.
Ans – DDT (dichloro diphenyl trichloroethane) was developed as the first of the modern synthetic insecticides in the 1940s. It was initially used with great effect to combat malaria, typhus, and the other insect-borne human diseases among both military and civilian populations.
(c) Tetrachloromethane
Ans – Tetrachloromethane, also known as carbon tetrachloride. It is a non-flammable, colourless liquid with a sweet chloroform-like smell that can be detected at low levels.
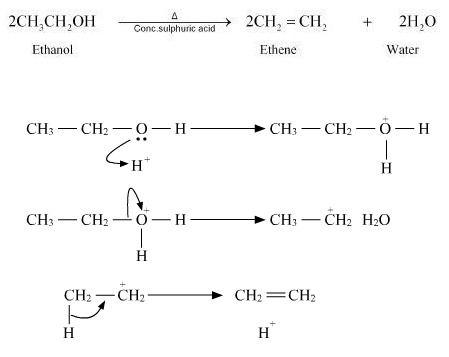
Q15. Write three steps mechanism of acid dehydration of ethanol to yield ethene.
Ans – Step I : Protonation of oxygen atom of -OH group.
Step II : Loss of a molecule of water to form carbonium ion.
Step III : Deprotonation to form carbon carbon double bond.
Q16.(i) Write short note on coupling reaction.
Ans – Coupling reaction is a class of organic reaction in which two chemical species are joined together with the help of a metal catalyst.
(ii) Why Gabriel phthalimide synthesis is preferred for synthesizing primary amines ?
Ans – Gabriel phthalimide reaction gives pure primary amines without any contamination of secondary and tertiary amines. Therefore, it is preferred for synthesising primary amines.
Q17.(i) What is the basic structural difference between starch and cellulose ?
Ans – Starches and cellulose are made of glucose molecules. Starch is a branched polymer, while cellulose is a linear polymer. This difference in structure makes starch more easily digestible by humans and animals, while cellulose is not digestible.
(ii) What are non-essential amino acid ?
Ans – Non-essential amino acids are amino acids that can be synthesized by the human body and do not need to be obtained through the diet. The human body can produce these amino acids using other amino acids or molecules. There are 11 non-essential amino acids : alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Q18.(i) Explain the both laws of Faraday Electrolysis.
Ans – The two laws of Faraday electrolysis are :
Faraday’s First Law – This law states that the amount of a substance produced during electrolysis is directly proportional to the amount of electricity passed through the electrolyte.
Mathematically, this can be expressed as :
m = zIt, where m is the mass of the substance produced, z is the number of electrons required for the reaction, I is the current, t is the time for which the current is passed.
Faraday’s Second Law – This law states that when the same amount of electricity is passed through different electrolytes, the masses of the substances produced at the electrodes are directly proportional to their chemical equivalent weights.
Mathematically, this can be expressed as :
m1/m2 = E1/E2, where m1 and m2 are the masses of the substances produced, E1 and E2 are their respective chemical equivalent weights.
(ii) A solution of Ni(NO3)2 is electrolysed between platinum electrode using a current of 5 ampere for 20 min., What mass of Ni is deposited at cathode ? (Ni = 58.7)
Ans – Current (I) = 5 amperes
Time = 20 × 60 = 1200 seconds
Charge = current x time = 5 x 1200 = 6000 C
According to the reaction,
Na²+(aq.) + 2e- → Ni(s)
Nickel deposit by (2×96487)C = 58.7 g
Nickel deposit by 6000 C = (58.7×6000) ÷ (2×96487) = 1.825 g
Hence 1.825g of nickel will be deposit at the cathode.
OR
(i) What is electrochemical series? Give its applications.
Ans – An electrochemical series is a activity series, in which elements are arranged in an increasing or decreasing order of their standard electrode potential. Electrochemical cells are used in torches, digital watches, military applications, corrosion protection, etc.
(ii) If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire ?
Ans – Current (I) = 0.5 A
Time (t) = 2 hrs = 2 × 60 × 60 = 7200 seconds
Charge (Q) = I × t = 0.5 × 7200 = 3600 C
Numbers of electrons = (Total Charge) ÷ (Charge of 1 electron)
No. of electrons = 3600 ÷ (1.6×10-¹⁹) = 2.25 × 10²² electrons
Q19. Explain giving reason :
(i) Transition metal and many of their compounds show paramagnetic behaviour.
Ans – This is due to presence of one or more unpaired electrons in d subshell.
(ii) The enthalpy of atomization of the transition metal are high.
Ans – This is due to high effective nuclear charge and large number of valence electrons. This results in formation of strong metallic bonds.
(iii) The transition metal generally forms coloured compounds.
Ans – This is due to d-d transition of unpaired electrons. In presence of ligands, the d orbitals split into two sets. Transition metal ions absorb radiation of a particular wavelength and reflect the remaining. This imparts colour.
(iv) Transition metal acts as good catalyst.
Ans – Transition metals show variable oxidation states and forms complexes. They form unstable intermediate compounds. They provide a new path with lower activation energy of reaction. They also provide a suitable surface for the reaction to occur.
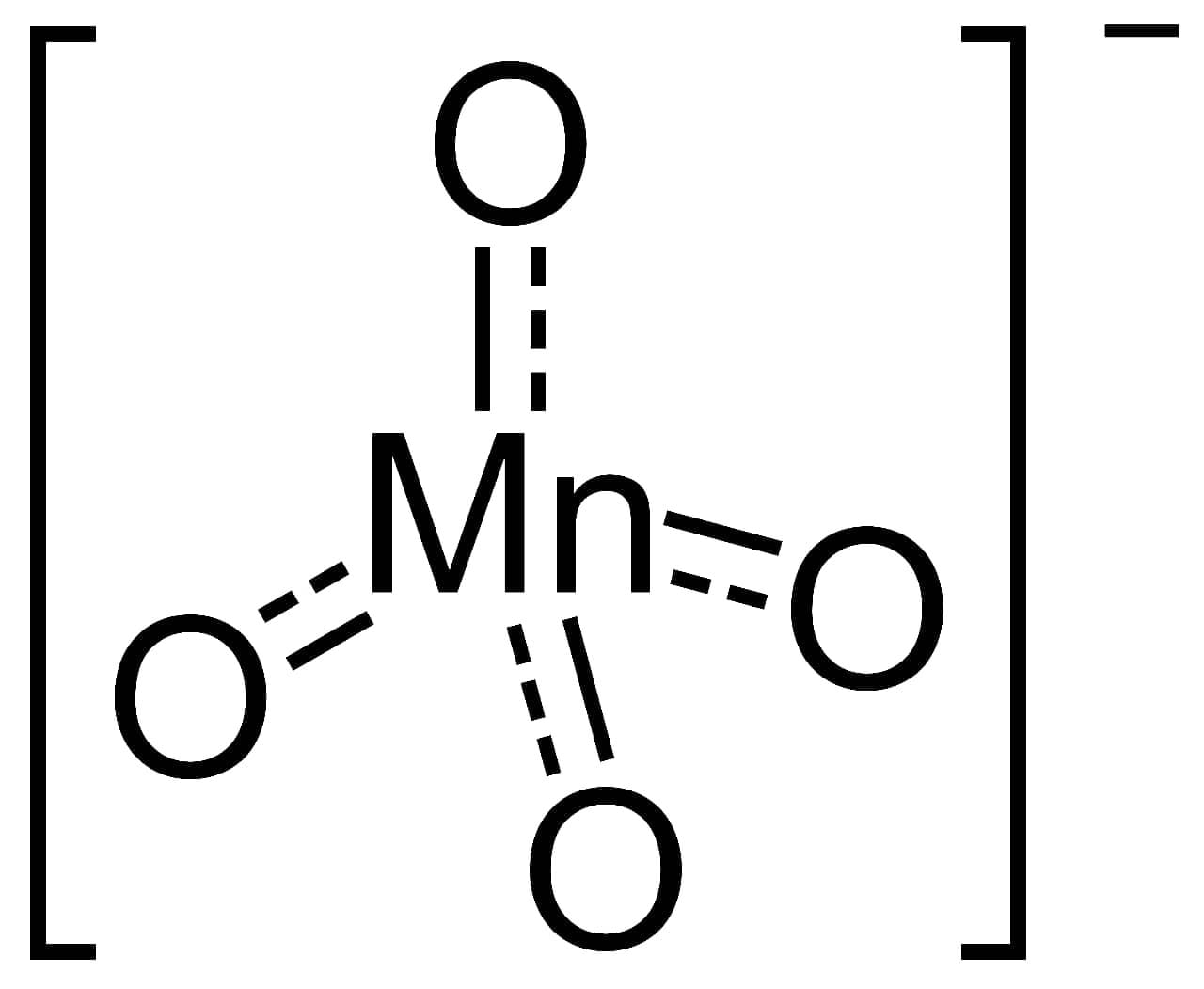
(v) Draw the structure of MnO4– Ion.
Ans :
OR
(i) Describe the preparation of KMnO4 from Pyrolusite ore.
Ans – Potassium Permanganate (KMnO4) is prepared from Pyrolusite ore (MnO2). The finely powdered Pyrolusite ore (MnO2) is fused with an alkali metal hydroxide like KOH in the presence of air or an oxidizing agent like KNO3. The oxidizing agent oxidizes pyrolusite to give the dark green potassium Manganate (K2MnO4). Potassium manganate undergoes different reactions in a neutral or acidic solution to give potassium permanganate.
2MnO2(s) + 4KOH(s) + O2(g) → 2K2MnO4(s) + 2H2O(l)
2K2MnO4(s) + 2H2O(aq) + Electrolysis → 2KMnO4(s) + 2KOH(s) + H2(g)
(ii) What are alloys ? Give two examples.
Ans – Alloys are metallic substances made by mixing two or more elements, at least one of which is a metal. The resulting material has properties that are different from those of its constituent elements, such as improved strength, durability, and resistance to corrosion.
For example, Brass is a mixture of copper and zinc; Stainless steel is mixture of iron, carbon, chromium and nickel.
Q20.(a) Describe the following :
(i) Cannizaro Reaction
Ans – Aldehydes which do not contain hydrogen when treated with a concentrated solution of an alkali undergo self oxidation-reduction. As a result, one molecule of aldehyde is reduced to corresponding alcohol while the other molecule is oxidized to the corresponding acid. This reaction is called Cannizzaro reaction.
(ii) Decarboxylation
Ans – Decarboxylation is a chemical reaction that involves the removal of a carboxyl group (-COOH) from a molecule, resulting in the formation of a new compound.
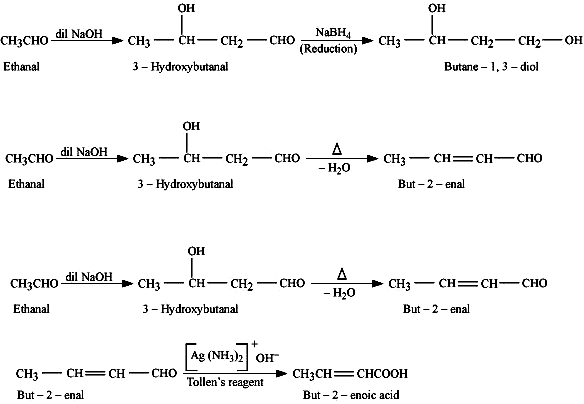
(b) Convert Ethanal into following :
(i) Ethanal to Butane-1,3-diol
(ii) Ethanal to But-2-enal
(iii) Ethanal to But-2-enoic acid
Ans –
OR
Describe the reaction with the help of equation :
(i) Cross Aldol condensation reaction
Ans – When aldol condensation is carried out between two different aldehydes and or ketones, it is called cross aldol condensation. If both the reactants contain α-hydrogens, four compounds are obtained as products. For example, ethanal and propanal react to give four products.
(ii) Hell-Volhard-Zelinsky reaction
Ans – The Hell-Volhard-Zelinsky reaction, also known as HVZ reaction. This reaction involves alpha bromination of carboxylic acids. Carboxylic acids react with chlorine or bromine in presence of small amount of red phosphorous to give alpha halo carboxylic acids.
(iii) Esterification
Ans – Esterification is the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR) and water; or a chemical reaction resulting in the formation of at least one ester product.
SET–B
Q1.(i) The Vant Hoff’s factor (i) for K2SO4 is :
(A) 1
(B) 2
(C) 0
(D) 3
Ans : (D) 3
(ii) Fused NaCl on electrolysis gives on Cathode is :
(A) Chlorine
(B) Sodium
(C) Sodium Amalgam
(D) Hydrogen
Ans : (B) Sodium
(iii) A reaction is second order with respect to reactant. How is rate of reaction affected if concentration of reactant is reduced to half ?
(A) 4 times
(B) 2 times
(C) 1/4 times
(D) 8 times
Ans : (C) 1/4 times
Rate, R = k[A]²
R’ = k[½A]² = k[¼A²] = ¼k[A]² = ¼R
(iv) What is the co-ordination nuumber of cobalt in the [CoCl2(en)2]Cl compound ?
(A) 6
(B) 4
(C) 8
(D) 2
Ans : (A) 6
(v) A tertiary alkyl halide would prefer to undergo :
(A) SN²
(B) Elimination
(C) Addition
(D) SN¹
Ans : (D) SN¹
(vi) In the reaction CH3CH2OH + conc.H2SO4 + 413K → A; A will be :
(A) CH3CH2CH2CH3
(B) CH3CH2OCH2CH3
(C) CH3CH2OCH3
(D) CH2=CH2
Ans : (B) CH3CH2OCH2CH3
(vii) IUPAC name of compound HOOC–COOH is …………..
Ans : ethan-1,2-dioc acid or Ethanedioic acid (Oxalic acid)
(viii) Hofmann bromamide degradation reaction involve :
(A) C6H5NH2
(B) C6H5CONH2
(C) C6H5NO2
(D) C6H5OH
Ans : (B) C6H5CONH2
(ix) Glycogen is an example of :
(A) Protein
(B) Polysaccharide
(C) Monosaccharide
(D) Disaccharide
Ans : (B) Polysaccharide
(x) Order of reaction is ……….., when K = 3 × 10-⁴ L mol-¹ s-¹.
Ans : Second Order
(xi) Complete the following reaction :
RCH2OH + CrO3(anhydrous) →
Ans : RCHO
(xii) Deficiency of Vitamin ‘A’ causes …………
Ans : Night blindness
(xiii) What is the cause of elevation in boiling point ?
Ans : When a non-volatile solute is added to a solvent, the vapour pressure of the resulting solution is lower than that of the pure solvent. Therefore, a greater amount of heat must be supplied to the solution for it to boil. This increase in the boiling point of the solution is the boiling point elevation.
(xiv) Write IUPAC name of [Co(NH3)6]Cl3 compound.
Ans : hexaammine cobalt (III) chloride
(xv) Name the reagent used in dehydration of Ethanol into Ethene.
Ans : concentrated sulphuric acid (conc.H2SO4)
Q2. Calculate the molarity of a solution containing 5.6g KOH in 500ml of solution.
Ans –
Q3. Define order of reaction and instantaneous rate of reaction.
Ans –
Q4. Explain the following :
(i) Ambident Ligands
Ans –
(ii) Co-ordination Number
Ans –
Q5. In the following pairs of halogen compounds which compound undergoes faster SN¹ reaction ?
Ans –
Q6. Why the enthalpies of atomization of the transition metals are high ?
Ans –
Q7. Explain with example Williamson Ether Synthesis.
Ans –
Q8. Describe the following :
(i) Cannizaro reaction
Ans –
(ii) Acetylation
Ans –
Q9. Give one chemical test to distinguish between secondary and tertiary amines.
Ans –
Q10.(i) Define mole fraction and mass percentage.
Ans –
(ii) Give an example of a solid solution in which the solute is a gas.
Ans –
Q11. How much charge required in Coulomb for the following reductions or oxidations ?
(i) 1 mole of Cu²+ to Cu
Ans –
(ii) 1 mole FeO to Fe2O3
Ans –
(iii) 1 mole of MnO4²- to MnO2
Ans –
Q12. For a first order reaction, show that time required for 99% completion is twice the time required for completion of 90% of the reaction.
Ans –
Q13. Explain why [NiCl4]²- is paramagnetic while [Ni(CO)2] is diamagnetic though both are tetrahedral ?
Ans –
Q14.(i) What are Ambident Nucleophiles, explain with an example ?
Ans –
(ii) Give the uses of :
(a) Freon
Ans –
(b) D.D.T.
Ans –
Q15. Why ortho-nitrophenol and para-nitrophenol are more acidic then phenol? Draw the resonance structure of phenoxide ions.
Ans –
Q16.(i) Write short note on carbylamine reaction.
Ans –
(ii) Why ethylamine is soluble in water whereas aniline is not ?
Ans –
Q17.(i) Give differences between RNA and DNA.
Ans –
(ii) What are Biomolecules? Give two examples.
Ans –
Q18.(i) What is Kohlrausch law? Discuss its applications.
Ans –
(ii) A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at the cathode ? (Cu = 63.5)
Ans –
OR
(i) What is Corrosion? Give factors which promotes corrosion and name the methods to prevent corrosion.
Ans –
(ii) The molar conductivity of 0.02 mol L-¹ methanoic acid is 46.1 S cm² mol-¹. Calculate its degree of dissociation. Given λ°(H+) = 349.6 S cm² mol-¹ and λ° (HCOO-) = 54.6 S cm² mol-¹.
Ans –
Q19.(i) Describe the oxidising action of K2Cr2O7 and the ionic equations for its reaction with : (a) iodide ion (b) iron(II) solution (c) H2S.
Ans –
(ii) Calculate magnetic moment of a trivalent ion in aqueous solution with atomic number 27.
Ans –
OR
(i) What is lanthanoid contraction? Give its causes and consequences.
Ans –
(ii) Why do transition elements exhibit tendency for complex formation? Explain with example.
Ans –
Q20. What is meant by following term’s, give an example of the reaction in each case ?
(i) Cynohydrin
Ans –
(ii) 2, 4, D.N.P.
Ans –
(iii) Kolbe’s Electrolysis
Ans –
(iv) Hemi-acetal
Ans –
(v) Ketal
Ans –
OR
Describe with equation :
(i) Wolff-Kishner reduction
Ans –
(ii) Aldol condensation reaction
Ans –
(iii) Decarboxylation reaction
Ans –
SET–C
Q1.
