Haryana Board (HBSE) Class 12 Chemistry Half Yearly Question Paper 2024 PDF Download. HBSE Class 12 Chemistry Half Yearly Question Paper 2024. Haryana Board Class 12 Chemistry Half Yearly Exam 2024. HBSE Class 12th Chemistry Half Yearly Paper 2024 Answer. Haryana Board Class 12 Half Yearly Paper PDF Download. Haryana Board Class 12 Chemistry Half Yearly Paper 2024 Solution. हरियाणा बोर्ड कक्षा 12 रसायन विज्ञान अर्धवार्षिक पेपर 2024.
HBSE Class 12 Chemistry Half Yearly Question Paper 2024 Answer Key
Instructions :
• All questions are compulsory.
• Questions (1-9) carry 1 mark each.
• Questions (10-12) carry 2 marks each.
• Questions (13-14) carry 3 marks each.
• Question (15) case study, carry 4 marks.
• Questions (16-17) carry 5 marks each.
1. Which of the following cannot be the molecularity of a chemical reaction?
(a) 2
(b) 1
(c) 0
(d) 3
Answer – (c) 0
2. Which of the following does not exhibit variable oxidation state?
(a) Sc
(b) Cu
(c) Ti
(d) Fe
Answer – (a) Sc (scandium, Z=21)
3. The stability of ferric ion is due to :
(a) Half-filled d-orbitals
(b) completely filled f-orbitals
(c) Half-filled f-orbitals
(d) completely filled d-orbitals
Answer – (a) Half-filled d-orbitals
4. The osmotic pressure of fluid inside human blood cell is equivalent to that of :
(a) 9.0% (mass/volume) NaCl
(b) 0.9% (mass/volume) NaCl
(c) 1.9% (mass/volume) NaCl
(d) None of these
Answer – (b) 0.9% (mass/volume) NaCl
5. If the molality of a dilute solution is doubled, then the value of molal depression constant will be ………….
Answer – remain unchanged
6. If the rate constant of a reaction is 0.9 mol L–1 Sec–1, then it will be a …………. order reaction.
Answer – Zero
7. The van’t hoff factor (i) for a dilute solution of K3[Fe(CN)6] is ……..
Answer : 4
8. Write the SI unit of molar conductivity.
Answer : S m2 mol–1 (Siemen metre square mol inverse)
9. Assertion (A) : Half-life of a first order reaction is variable.
Reason (R) : For first order reaction, half-life is independent of initial concentration of reactant.
Answer – Assertion (A) is false but Reason (R) is true.
10. What are the factors affecting rate of reaction?
Answer – Concentration of reactants & pressure in case of gases, temperature, and catalyst.
11. Define Kohlrausch law.
Answer – The law states that limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
12. Haloarenes are less reactive than haloalkanes why?
Answer – In haloarenes there is double bond character between carbon and halogen due to resonance effect which makes him less reactive. In benzene carbon being sp2 hybridised which is smaller in size than sp3 present in haloalkanes. So, C–Cl bond in aryl halides is shorter and stronger.
13. Represent the cell in which the following reaction takes place
Mg(s) + 2Ag+ (0.0001M) → Mg2+ (0.130M) + 2Ag(s)
Calculate its Ecell if Eocell = 3.17 V.
Answer : Ecell = Eocell – 0.0591/n log[Mg2+/(Ag+)2]
Ecell = 3.17 – 0.0591/2 log[0.130/(0.0001)2]
Ecell = 3.17 – 0.21 = 2.96 V
14. Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Answer – The shielding effect of 5f orbitals is poorer than the shielding effect of 4f orbitals. Due to this, the valence shell electrons of actinide experience greater effective nuclear charge than that experienced by lanthanides. Hence, actinoid contraction is greater than lanthanoid contraction.
15. CASE STUDY : Transition elements have partly filled d-orbitals in their normal oxidation states or in their common oxidation states. There are four series in transition elements and these are 3d-series (first transition series), 4d-series (second transition series), 5d-series (third transition series) and 6d-series (fourth transition series). Each series contains ten elements. Elements of group 12 are not considered as transition elements as these have fully-filled d-subshells. Transition elements show all the characteristics of metals. These elements show variable oxidation states, form coloured ions, form complexes from alloys and interstitial compounds. They show high enthalpies of atomisation, show catalytic properties and magnetic moment.
Questions :
(a) Why Zn, Cd and Hg are not considered as transition elements?
Answer – Transition metals are defined as the elements having incompletely filled d-orbitals. Since Zn, Cd and Hg have completely filled d-orbital, they are not regarded as transition elements.
(b) Which element of d-block shows maximum oxidation state?
Answer – Manganese
(c) Why transition elements show variable oxidation states?
Answer – Transition elements show variable state oxidation in their compounds because there is a very small energy difference between (n–1)d and ns orbitals. As a result, electrons of (n–1)d orbitals as well as ns-orbitals take part in bond formation. Thus, transition elements have variable oxidation states.
(d) Calculate the magnetic moment of a divalent ion in its aqueous solution if its atomic number is 25.
Answer : Magnetic moment (µ) = √n(n+2)
= √5(5+2) = √35 = 5.92 BM
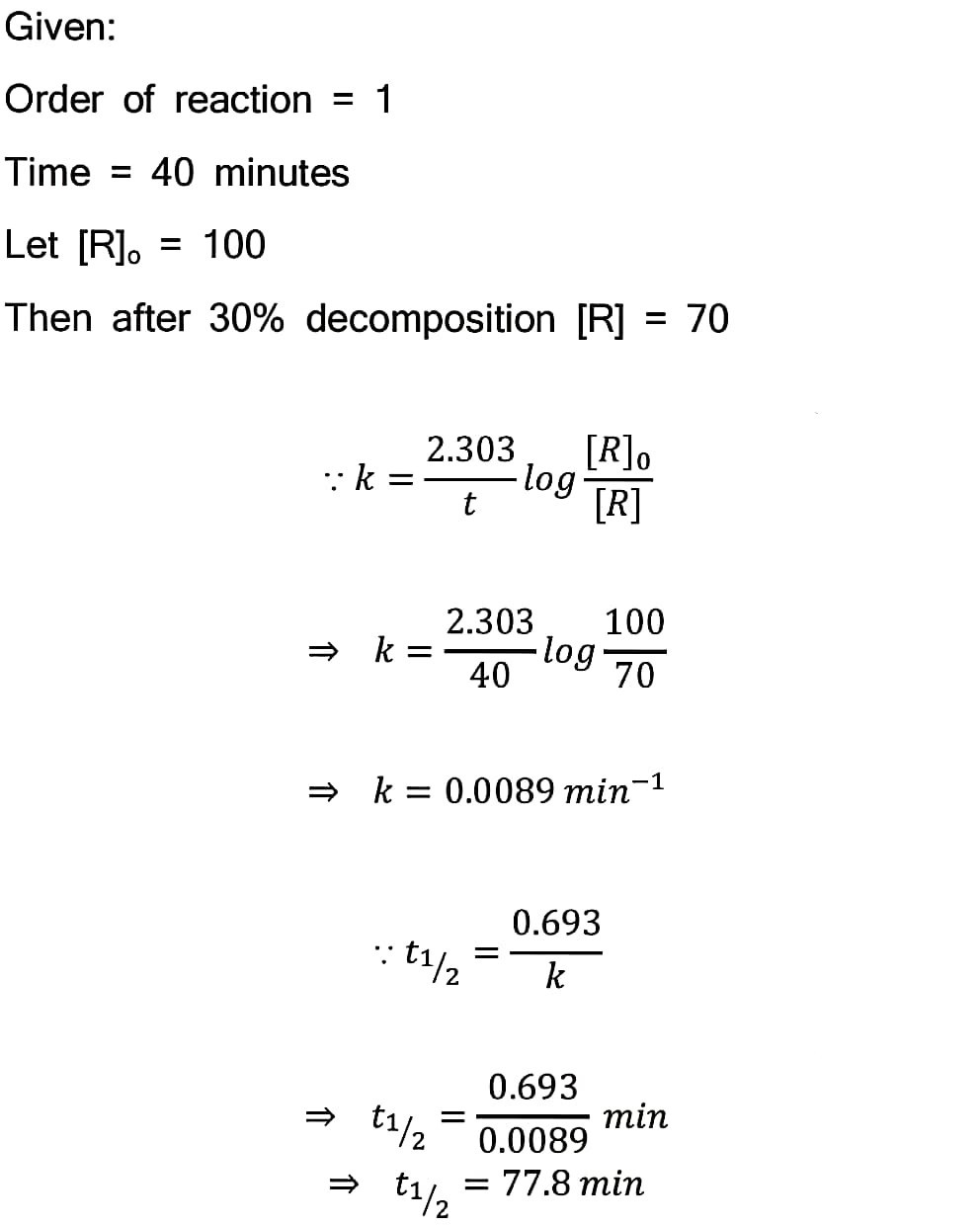
16. A first order reaction takes 40 min for 30% decomposition. Calculate t1/2.
Answer –
17. State Faraday’s law of electrolysis.
Answer : First Law – The amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolyte.
• Second Law – The amounts of different substances liberated by the same quantity of electricity passing through the electrolytic solution are proportional to their chemical equivalent weights.
