Haryana Board (HBSE) Class 10 Science Question Paper 2023 Answer Key. Haryana Board Class 10th Science Solved Question Paper 2023 PDF Download. HBSE Class 10 Science Previous Year Question Paper with Answer. HBSE 10th Science Solved Question Paper 2023. HBSE 10th Class Science Solved Question Paper 2023.
HBSE Class 10 Science Question Paper 2023 Answer Key (All Sets – A,B,C,D)
SET–A
(Physics)
Q1. The least distance of distinct vision for normal eye is :
(A) 2.5 cm
(B) 25 cm
(C) 25 m
(D) 2.5 m
Ans – (B) 25cm
Q2. Which of the following is not a natural resource ?
(A) Water
(B) Soil
(C) Electricity
(D) Air
Ans – (C) Electricity
Q3. SI unit of electric current is :
(A) Ampere
(B) Newton
(C) Volt
(D) Joule
Ans – (A) Ampere
Q4. Tehri dam is built on which river ?
(A) Narmada
(B) Godavari
(C) Ganga
(D) Yamuna
Ans – (C) Ganga
Q5. Why do stars twinkle ?
Ans – Due to atmospheric refraction of light.
Q6. What are the characteristic of a good source of energy ?
Ans – (i) It should be a sustainable and renewable source of energy.
(ii) It should have a high calorific value.
(iii) It should be easily accessible and provide energy for the maximum period of time.
(iv) It should not cause pollution.
Q7. An electric current of 0.5 A flows through the filament of an electric bulb for 5 minutes. What will be the electric charge flowing through that wire ?
Ans : Current (i) = 0.5, t = 5 min = 5 × 60 = 300 second
Charge (Q) = i × t = 0.5 × 300 = 150 Coulomb
Q8. What is an electric motor ? Draw its labelled diagram and write its principle.
Ans : An electric motor is a device that converts electrical energy into mechanical energy.
Principle – It works on the principle of electromagnetic induction, where a magnetic field is created by passing an electric current through a coil of wire, which then interacts with a permanent magnet to produce rotational motion.
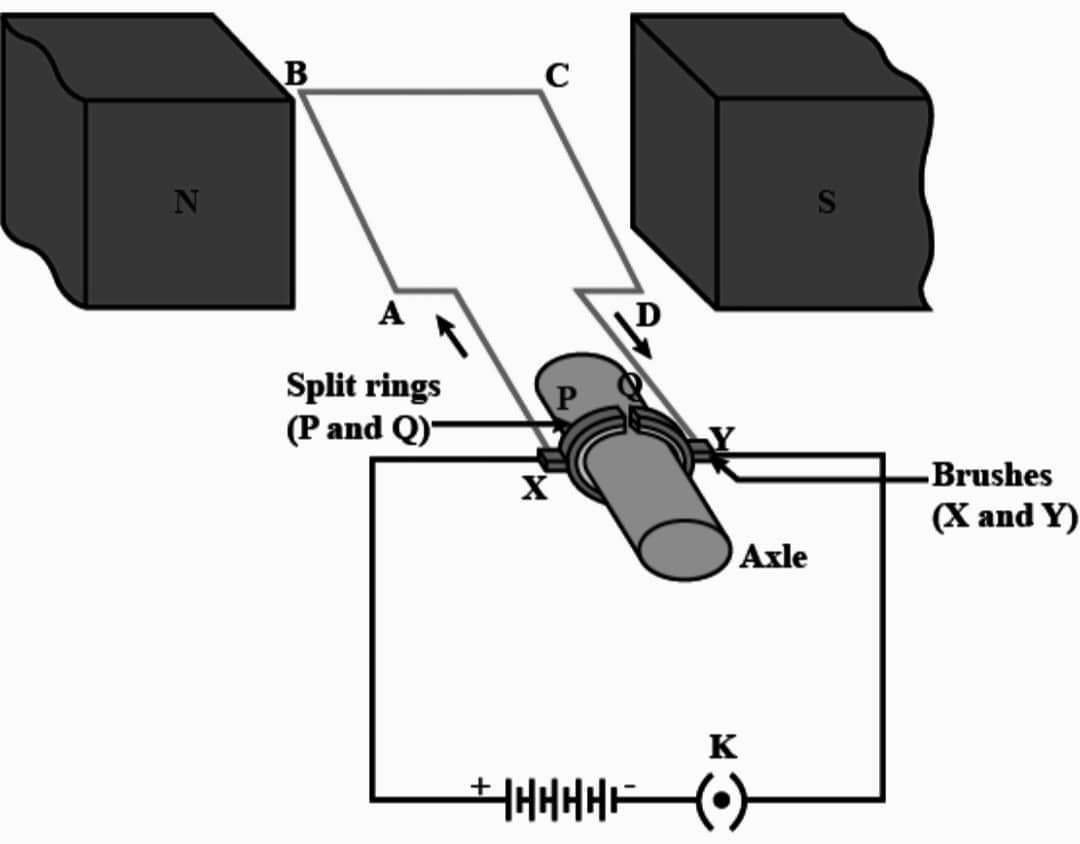
Working – Current in the coil ABCD enters from the source battery through conducting brush X and flow back to the battery through brush Y. Notice that the current in the arm AB of the coil flows from A to B. In arm CD it flows from C to D that is opposite to the direction of current through arm AB on applying Fleming’s left hand rule for the direction of force on a current-carrying conductor in a magnetic field. We find that the force acting on arm AB pushes it downwards while the force acting on arm CD pushes it upwards. Thus the coil and the Axle O, mounted free to turn about an axis, rotate anti-clockwise at half rotation. Q makes contact with the brush X and P with brush Y. Therefore the current in the coil gets reversed and flows along the path DCBA. The reversal of current also reverses the direction of force acting on the two arms AB and CD. Thus the arm AB of the coil that was earlier pushed down, is now pushed up and the arm CD previously pushed up is pushed down. There is a continuous rotation of the coil and to the axle.
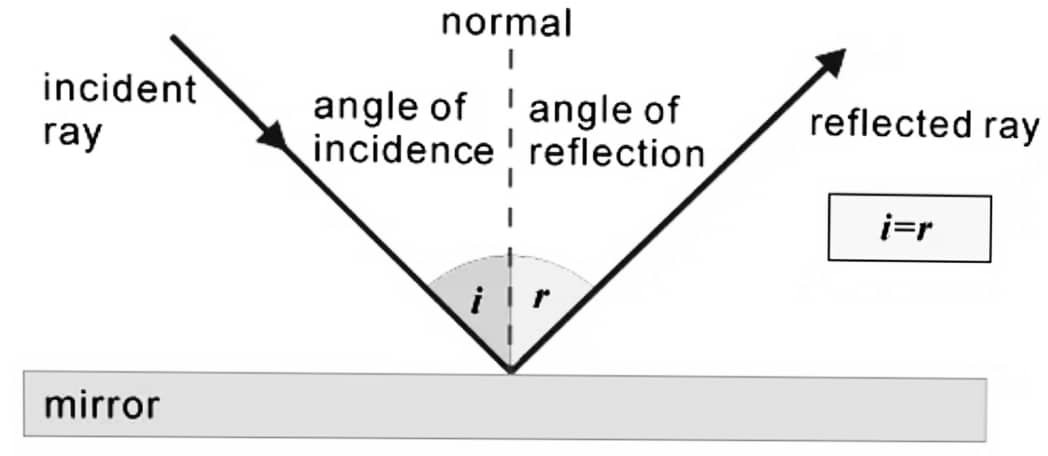
Q9.(a) Write the laws of reflection of light.
Ans – The incident ray, the reflected ray and the normal at the point of incidence, all lie in the same plane.
The angle of incidence is equal to the angle of reflection. i.e. ∠i = ∠r
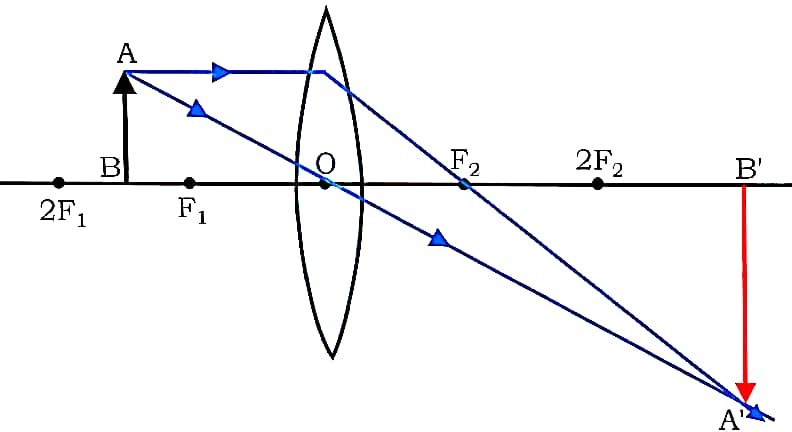
(b) Draw a ray diagram to show the image formed by a convex lens when the object is placed between principal focus (F1) and 2F1.
Ans – The image is real, inverted, larger than object.
(c) What is the focal length of convex mirror, whose radius of curvature is 36 cm ?
Ans : Focal Length (F) = R/2 = 36/2 = 18 cm
OR
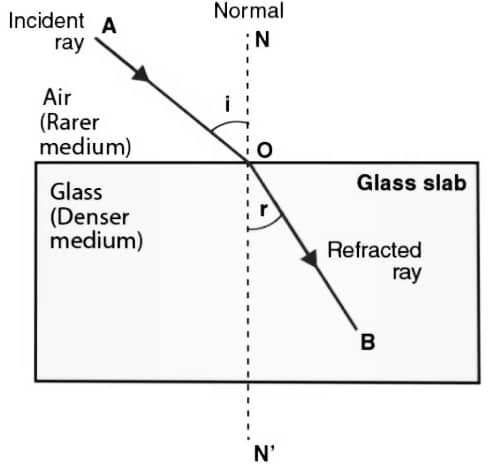
(a) Write the laws of refraction of light.
Ans – The incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
The ratio of the sine of the angle of incidence (i) to the sine of the angle of refraction (r) is constant for the pair of given medium. i.e sini/sinr = constant
(b) State the types of mirror used and give reason to justify your answer :
(i) headlights in car
Ans – Concave mirror is used in the headlights of cars to produce a parallel beam of light covering a longer distance on the road.
(ii) rear view mirror in any vehicle
Ans – Convex mirror is used in rear view mirrors of motor vehicles because they give an erect, virtual, full size diminished image of distant objects with a wider field of view. Thus, convex mirrors enable the driver to view much larger area.
(c) Write the lens formula.
Ans – Lens formula is 1/f = 1/v – 1/u
Here, focal length (f), image distance (v), object distance (u)
(Chemistry)
Q10. In which of the following a balanced chemical equation with state symbols is correct ?
(A) CO(g) + 2H2(g) → CH3OH(g)
(B) CO(l) + 2H2(g) → CH3OH(l)
(C) CO(g) + 2H2(g) + 340atm → CH3OH(l)
(D) CO(g) + 2H2(l) + 340°C → CH3OH(l)
Ans – (C) CO(g) + 2H2(g) + 340atm → CH3OH(l)
Q11. Which of the following forms the basis of the modern periodic table ?
(A) Atomic mass
(B) Atomic number
(C) Number of Nucleons
(D) Number of Neutrons
Ans – (B) Atomic number
Q12. What is chemical name of baking soda ?
(A) Sodium Carbonate
(B) Sodium hydrogen Carbonate
(C) Sodium Chloride
(D) Sodium Oxide
Ans – (B) Sodium hydrogen Carbonate (NaHCO3)
It is also known as Sodium bicarbonate.
Q13. What is the colour of Ferrous sulphate crystals? How does this colour change after heating ?
Ans – The colour of ferrous sulphate crystals (FeSO4.7H2O) is light Green. On heating, ferrous sulphate crystals lose water and anhydrous ferrous sulphate (FeSO4) is formed. So their colour changes from light green to white.
Q14. What is neutralization reaction, explain with example ?
Ans – When an acid and a base react to form water and salt it is called neutralization reaction. The resultant salt is neither acidic nor basic in nature i.e. it is Neutral.
Acid + Base → Salt + Water
For Example, when Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the resulting salt is sodium chloride (NaCl) and water.
HCl + NaOH → NaCl + H2O
Q15. Oxygen (Atomic No. 8) and Sulphur (Atomic No. 16) belongs to group 16 in periodic table. Give electronic configuration and which is more electronegative, why ?
Ans – Electronic configuration of Oxygen is (2, 6) and Sulphur is (2, 8, 6).
Oxygen is more electronegative than Sulphur. This is because Oxygen has a smaller atomic radius and higher effective nuclear charge than Sulphur, which makes it attract electrons more strongly towards itself.
Q16.(i) What are amphoteric oxides? Give examples.
Ans – Amphoteric oxides are those oxides that can act as both acidic and basic oxides depending on the reaction conditions.
Examples : Aluminum oxide (Al2O3), Zinc oxide (ZnO)
(ii) Explain the meaning of malleable and ductility with example.
Ans. Malleable – Substances that can be beaten into thin sheets. For example, Aluminium, Copper.
Ductile – Substances that can be drawn into thin wires. For example, Silver, Copper.
Q17.(a) Complete the following chemical reaction :
(i) CH3COOH + NaHCO3 →
Ans : CH3COONa + CO2 + H2O
(ii) Na + C2H5OH →
Ans : C2H5ONa + ½H2
(iii) C2H5OH + Hot Conc.H2SO4 →
Ans : CH2=CH2 (ethene)
(b)(i) Make a structure of Benzene.
Ans –
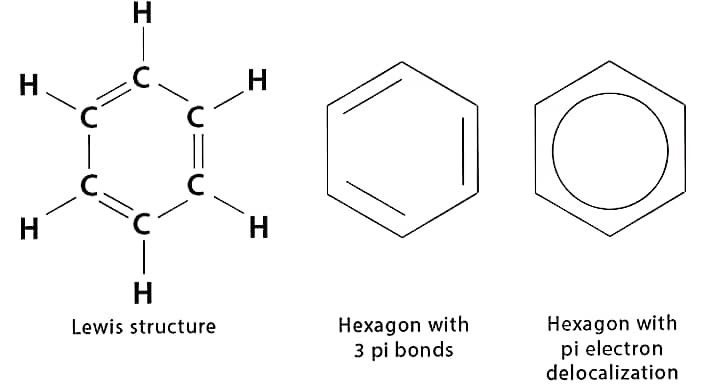
(ii) Write a short note on Micelles.
Ans – The unique orientation of soap/detergent molecules inside the water which prevents the contact of the hydrophobic (water fearing) part of the molecule with water is known as a micelle.
OR
(a) Write the names of following compounds :
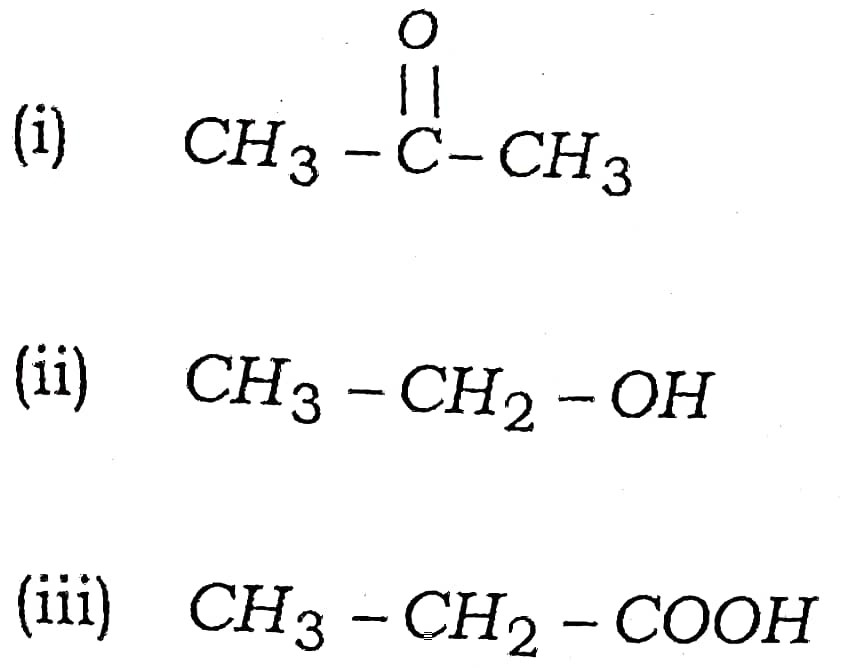
Ans –
(i) Propanone
(ii) Ethanol
(iii) Ethanoic acid
(b)(i) Explain addition reaction with example.
Ans – A chemical reaction where two or more reactants combine to form a single product is called an addition reaction.
Example : 3Fe + 2O2 → Fe3O4
(ii) Make a structure of Benzene.
Ans –

(Biology)
Q18. Which gland secrete Insulin ?
(A) Liver
(B) Adrenal
(C) Pineal
(D) Pancreas
Ans – (D) Pancreas
Q19. Multiple fission take place in :
(A) Amoeba
(B) Paramecium
(C) Leishmania
(D) Plasmodium
Ans – (D) Plasmodium
Q20. Which is a natural ecosystem ?
(A) Garden
(B) Crop fields
(C) Both (A) and (B)
(D) Pond
Ans – (D) Pond
Q21. Write full form of CFCs.
Ans – Chloro Fluoro Carbons
Q22. Which structure protects spinal cord ?
Ans – Vertebral Coulmn
Q23. What are the various parts of central nervous system? Write their functions.
Ans – The central nervous system (CNS) is consists of the brain and spinal cord.
The brain controls most bodily functions, including awareness, movements, sensations, thoughts, speech and memory.
The spinal cord carries messages back and forth between the brain and the nerves that run throughout the body.
Q24. Write about spore formation in Rhizopus.
Ans – Rhizopus is the species of a fungus. They reproduce asexually by the formation of the spores. The body of the fungus is composed of hyphae which develop the sporangium. The sporangium is a swollen structure at the tip of the filaments bearing the spores.
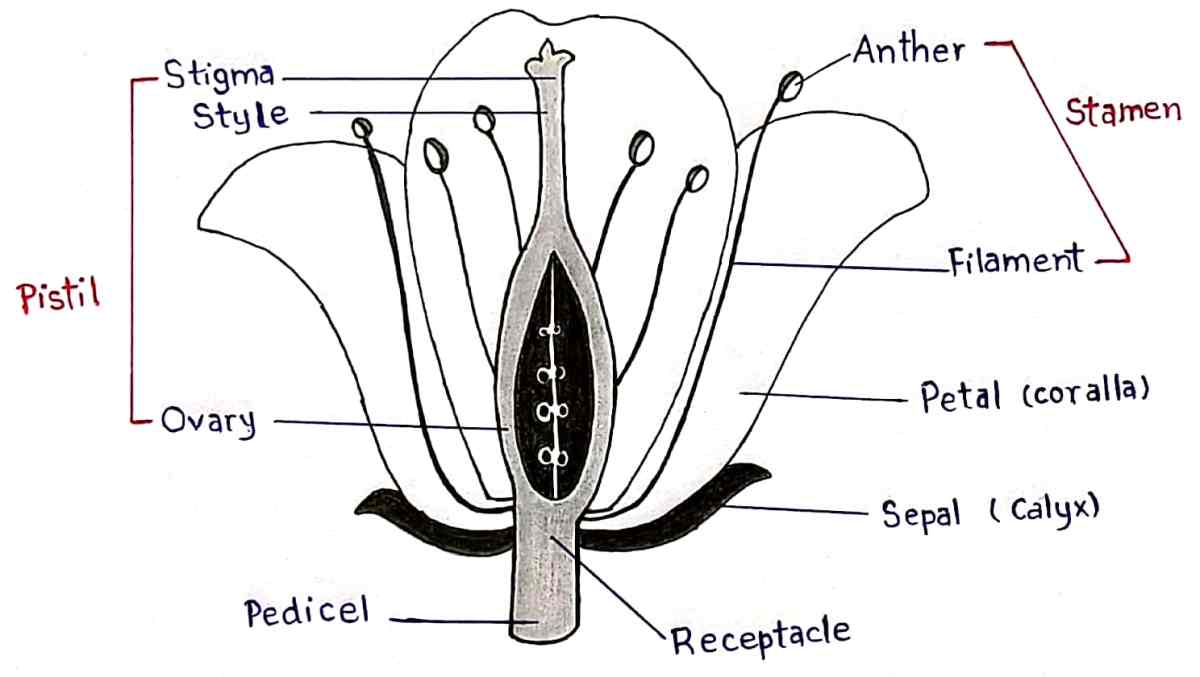
Q25. Discuss the structure of female reproductive part of a flower.
Ans : Stigma, Style and Overy
• Stigma – Receives the pollen during pollination.
• Style – A stalk that connects stigma to ovary.
• Ovary – Contains ovules which are the female reproductive cells (egg cells).
Q26. How do Mendel’s experiment show that traits are inherited independently ?
Ans – Mendel’s experiments with pea plants showed that traits are inherited independently through the process of segregation and independent assortment. He crossed pea plants with different traits, such as tall and short, and observed that the offspring inherited one allele from each parent for each trait. This is known as the law of segregation.
He then crossed pea plants with two different traits, such as seed color and seed shape, and observed that the inheritance of one trait did not affect the inheritance of the other trait. This is known as the law of independent assortment.
Overall, Mendel’s experiments demonstrated that traits are inherited independently of each other, which is now known as the principle of independent assortment.
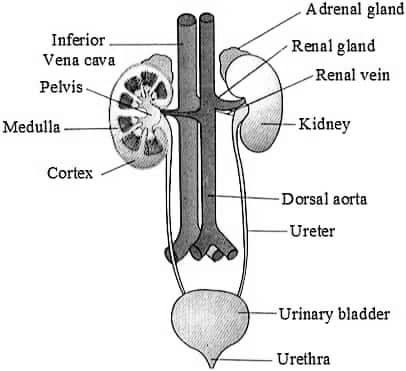
Q27. Explain the structure of human excretory system with the help of a well labelled diagram.
Ans : The human excretory system is made up of several organs that work together to rid the body of waste products. These organs include the kidneys, ureters, bladder, and urethra.
Kidneys – The kidneys are two bean-shaped organs located on either side of the spine, just above the waist. Inside the kidneys are tiny tubes called nephrons, which filter waste products from the blood. The kidneys also help regulate the balance of salt and water in the body.
Ureters – The ureters are two narrow tubes that carry urine from the kidneys to the bladder. They are about 25-30 cm in length.
Bladder – The bladder is a muscular sac that stores urine until it is ready to be expelled from the body. It is located in the pelvic region, just behind the pubic bone. The bladder can hold up to 500ml of urine.
Urethra – The urethra is a thin tube that carries urine from the bladder out of the body. In men, the urethra also carries semen out of the body. The length of the male urethra is much longer than that of females.
OR
How do living things get their food? Write various strategies.
Ans – Living things need food to obtain the energy and nutrients necessary for their survival, growth, and reproduction. Living things use a variety of strategies to obtain their food. Here are some examples :
Autotrophs – These are organisms that make their own food through photosynthesis. They convert energy from the sun into glucose, which they use as their source of energy.
Heterotrophs – These are organisms that cannot make their own food and depend on other sources for their nutrition.
Herbivores – These are animals that feed on plants exclusively. They have specialized teeth and digestive systems to help them digest plant material.
Carnivores – These are animals that feed on other animals. They have sharp teeth and strong jaws to help them hunt and consume their prey.
Omnivores – These are animals that feed on both plants and animals. They have a varied diet and can adapt to different food sources depending on availability.
Detritivores – These are organisms that feed on dead organic matter, such as fallen leaves or dead animals. They play an important role in decomposing organic matter and recycling nutrients back into the ecosystem.
Parasites – These are organisms that feed on a host organism, often causing harm or disease. They depend on their host for their nutrition and survival.
Symbiotic relationships – Some organisms have developed mutually beneficial relationships with other species. For example, some plants have evolved to depend on pollinators for reproduction, while the pollinators depend on the plants for food.
SET–B
(Physics)
Q1. The splitting of white light into seven colours is known as :
(A) Reflection
(B) Refraction
(C) Dispersion
(D) None of these
Ans – (C) Dispersion
Q2. Which of the following is/are a fossil fuel ?
(A) Coal
(B) Natural gas
(C) Petroleum
(D) All of the above
Ans – (D) All of the above
Q3. SI unit of electric charge is :
(A) Watt
(B) Ampere
(C) Coulomb
(D) Joule
Ans – (C) Coulomb
Q4. Chipko Andolan was started in :
(A) Uttarakhand
(B) Jharkhand
(C) Himachal
(D) Bihar
Ans – (A) Uttarakhand
Q5. Write ancient method of water harvesting in any two states of India.
Ans – Khadin and bawadi in Rajasthan, Bandharas in Maharashtra, Eri in Tamilnadu.
Q6. Explain why the planets do not twinkle ?
Ans – Planets do not twinkle because they appear larger in size than the stars as they are relatively closer to earth. Planets are not a source of light.
Q7. When a 12 V battery is connected across an unknown resistor there is a current of 2.5 mA in the circuit. Find the value of resistance of the resistor.
Ans – Here, V = 12V, I = 2.5mA = 2.5 × 10-³ A
V = IR
R = V/I = 12/(2.5×10-³) = 4.8 × 10³ Ω
Q8. What is an electric generator ? Draw its labelled diagram and write its principle.
Ans : An electric generator is a device that converts mechanical energy into electrical energy.
Principle – It works on the principle of electromagnetic induction, where a magnetic field is rotated inside a coil of wire, which then induces an electric current in the wire.
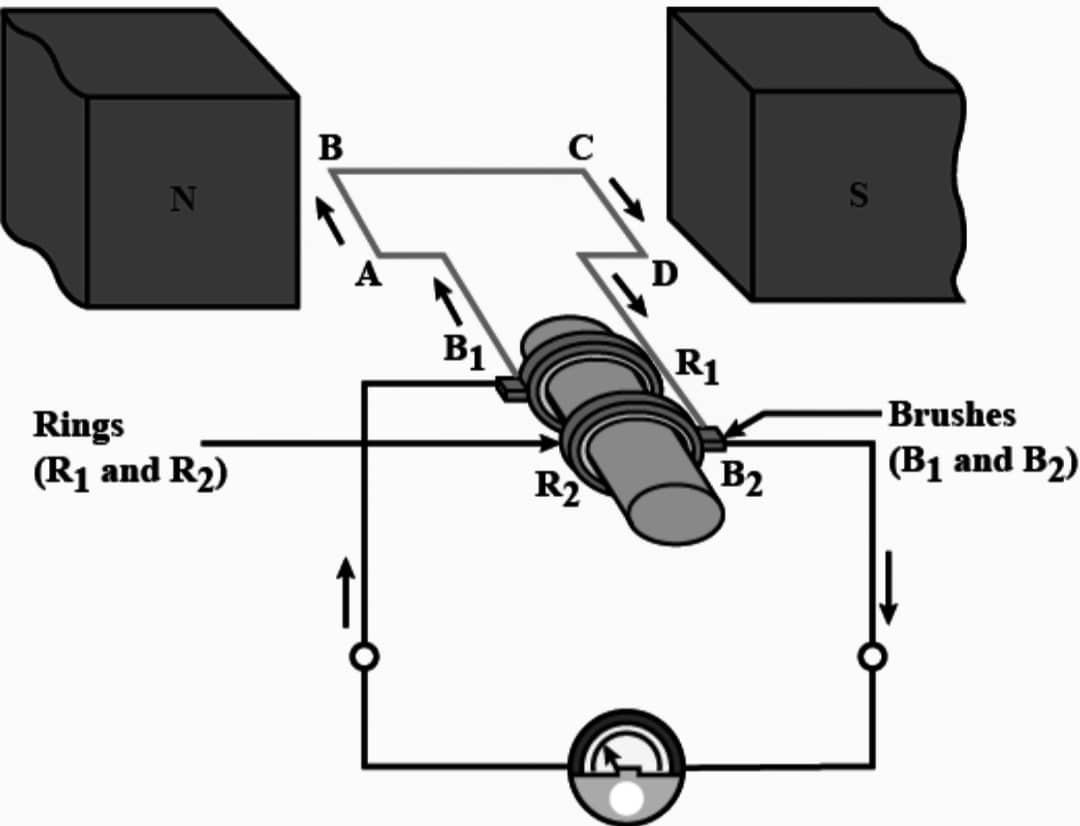
Working – When the axle attached to the two rings is rotated such that the arm AB moves up (and the arm CD moves down) in the magnetic field produced by the permanent magnet. Let us say the coil ABCD is rotated clockwise in the arrangement. By applying Fleming’s right hand rule, the induced currents are set up in these arms along the directions AB and CD. Thus an induced current flows in the direction ABCD. If there are larger number of turns in the coil, the current generated in each turn adds up to give a large current through the coil. This means that the current in the external circuit flows from B2 to B1. After half a rotation, arm CD starts moving up and AB moving down. As a result, the directions of the induced currents in both the arms changes, giving rise to the net induced current in the direction DCBA. The current in the external circuit now flows from B1 to B2. Thus after every half rotation the polarity of the current in the respective arms changes.
There are two brushes and in the electric generator, one brush is at all times in contact with the arm moving up in the field, while the other is in contact with the arm moving down. Because of these Brushes unidirectional current is produced. Function of brushes is to transfer the current from coil to load connected in the circuit of the electric generator.
Q9.(a) Light enters from air to glass having refractive index 1.50. What is the speed of light in glass? The speed of light in vacuum is 3×10⁸ m/s.
Ans : Speed of light in glass = Speed of light in vacuum ÷ Refractive index
v = c/n = (3×10⁸)/1.50 = 2 × 10⁸ ms-¹
(b) An object is situated at a position in between the main focus (F1) and 2F1 of a convex lens. Draw the ray diagram showing the position, size and nature of image.
Ans – The image is real, inverted, larger than object.

(c) Write the laws of refraction of light.
Ans – The incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
The ratio of the sine of the angle of incidence (i) to the sine of the angle of refraction (r) is constant for the pair of given medium. i.e sini/sinr = constant

OR
(a) Name the type of mirror used in the following situation and support your answer with reason :
(i) side/rear view mirror of a vehicle.
Ans – Convex mirror is used in rear view mirrors of motor vehicles because they give an erect, virtual, full size diminished image of distant objects with a wider field of view. Thus, convex mirrors enable the driver to view much larger area.
(ii) solar furnace
Ans – Concave mirror is used in solar furnace because they converge the parallel sun rays at a point. This helps to increase the temperature of the furnace.
(b) An object is placed at the centre of curvature (C) of a concave mirror. Draw the ray diagram to depict the position, size and nature of image formed.
Ans – The image is real, inverted, size equal to object.
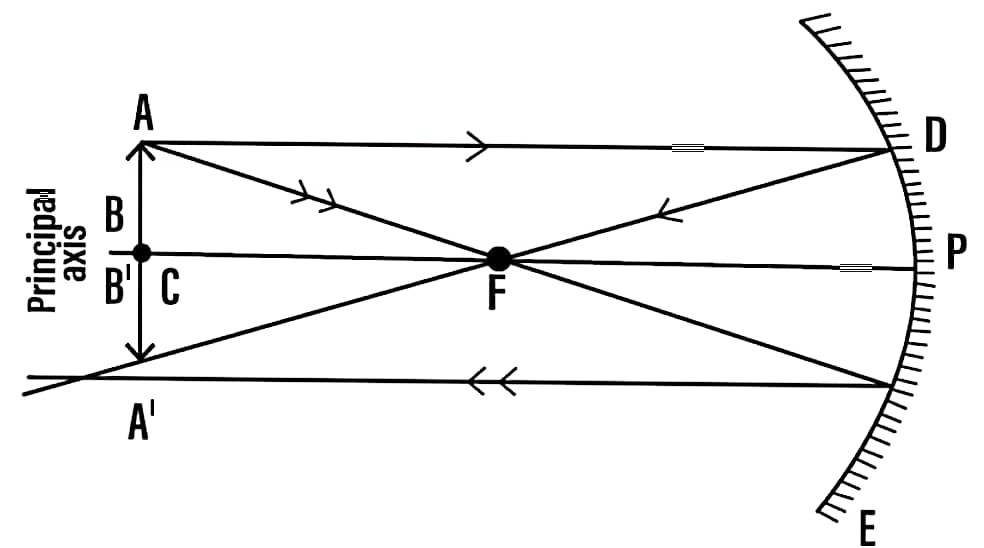
(c) Write the mirror formula.
Ans – Mirror formula is 1/f = 1/v + 1/u
Here, focal length (f), image distance (v), object distance (u)
(Chemistry)
Q10. The chemical formula of quick lime :
(A) CaO
(B) CaCO3
(C) Ca(OH)2
(D) CaOCl2
Ans – (A) CaO (Calcium oxide)
Q11. Which of the following is not a salt ?
(A) Baking Soda
(B) Washing Soda
(C) Vinegar
(D) Blue Vitriol
Ans – (C) Vinegar
Q12. How many elements in third and fifth period respectively in modern periodic table ?
(A) 8, 18
(B) 18, 32
(C) 8, 32
(D) 18, 18
Ans – (A) 8,18
Q13. What is redox reaction? Give an example.
Ans – The reactions in which oxidation and reduction reactions occur simultaneously are called redox reactions. The redox reactions are electron transfer reactions. Oxidation is the loss of electrons and reduction is the gain of electrons.
Example : Zn + Cu²+ → Zn²+ + Cu
Q14. Four solution A, B, C and D when tested with a universal indicator, showed pH as 1, 7, 6 and 13 respectively. Which solution is neutral, strongly alkaline, weak alkaline or weak acid and strongly acidic ?
Ans – The pH scale is a measure of acidity or alkalinity of a solution and ranges from 0 to 14. A pH of 7 is considered neutral, below 7 is acidic and above 7 is alkaline. The scale is logarithmic, meaning that each whole number increase or decrease represents a ten-fold increase or decrease in acidity or alkalinity.
Solution A(pH=1) is Strongly Acidic
Solution B(pH=7) is Neutral
Solution C(pH=6) is Weak Acid
Solution D(pH=13) is Strongly Alkaline
Q15. An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element ?
Ans – Atomic number is 17 (2+8+7) of Chlorine.
(b) To which of the following elements would it be chemically dissimilar? [Atomic no. are given in brackets, N(7), F(9), P(15), Br(35)]
Ans – Fluorine (F) has an atomic number of 9, which is close to Chlorine’s atomic number of 17. Both Chlorine and Fluorine belong to the same group (Group 17 or Halogens) in the periodic table and have similar chemical properties.
Therefore, Chlorine would be chemically similar to Fluorine and dissimilar to Nitrogen, Phosphorus, and Bromine.
16.(i) What are amphoteric oxides? Give example.
Ans – Amphoteric oxides are those oxides that can act as both acidic and basic oxides depending on the reaction conditions.
Examples : Aluminum oxide (Al2O3), Zinc oxide (ZnO)
(ii) Explain the meanings of malleable and ductility with example.
Ans : Malleable – Substances that can be beaten into thin sheets. For example, Aluminium, Copper.
Ductility – It is the physical property that substance can be drawn into thin wires. For example, Silver, Copper.
Q17. Write the names of following compounds :
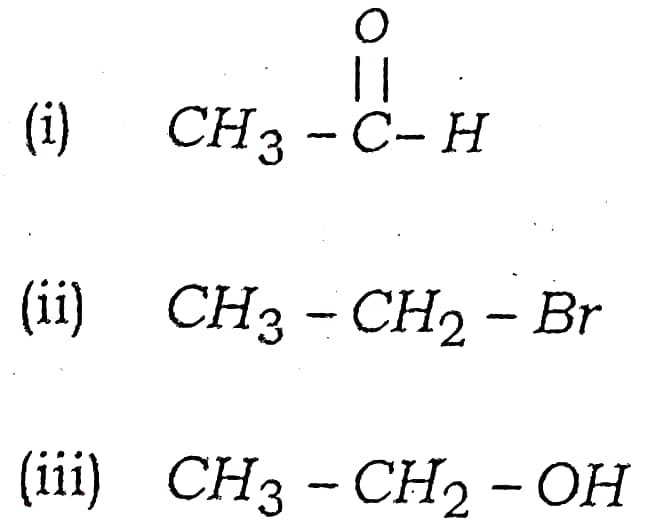
Ans –
(i) Ethanal
(ii) Bromoethane
(iii) Ethanol
(b) Identify alkanes, alkenes and alkynes in following :
C2H2, C2H4, C3H8, C4H10, C4H8, C5H8
Ans –
Alkanes (CnH2n+2) : C3H8 (propane), C4H10 (butane)
Alkenes (CnH2n) : C2H4 (ethylene), C4H8 (butene)
Alkynes (CnH2n-2) : C2H2 (acetylene), C5H8 (isoprene)
OR
(a) Write the names of following compounds :
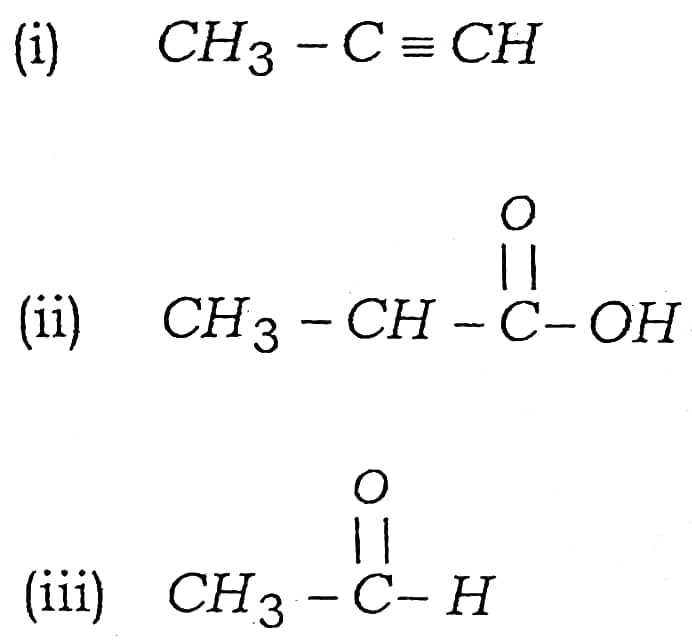
Ans –
(i) Propyne
(ii) Propanoic acid
(iii) Formaldehyde
(b)(i) Explain substitution reaction with example.
Ans – A substitution reaction is a type of chemical reaction where an atom or functional group of a molecule is replaced by another atom or functional group.
Example : CH4 + Cl2 → CH3Cl + HCl
(ii) Give structure formula of Butane.
Ans : CH3–CH2–CH2–CH3
(Biology)
Q18. Regeneration is found in :
(A) Hydra
(B) Planaria
(C) Both (A) and (B)
(D) None of these
Ans – (C) Both (A) and (B)
Q19. Birds are very closely related to :
(A) Amphibians
(B) Reptile
(C) Mammal
(D) None of these
Ans – (B) Reptile
Q20. Which of the following belongs to first trophic level ?
(A) Primary consumer
(B) Secondary consumer
(C) Tertiary consumer
(D) None of these
Ans – (D) None of these (‘Producer’ is belong to first trophic level)
Q21. Write scientific name of human.
Ans – Homo sapiens
Q22. What is the role of decomposers ?
Ans – To decompose or break down waste and dead organisms.
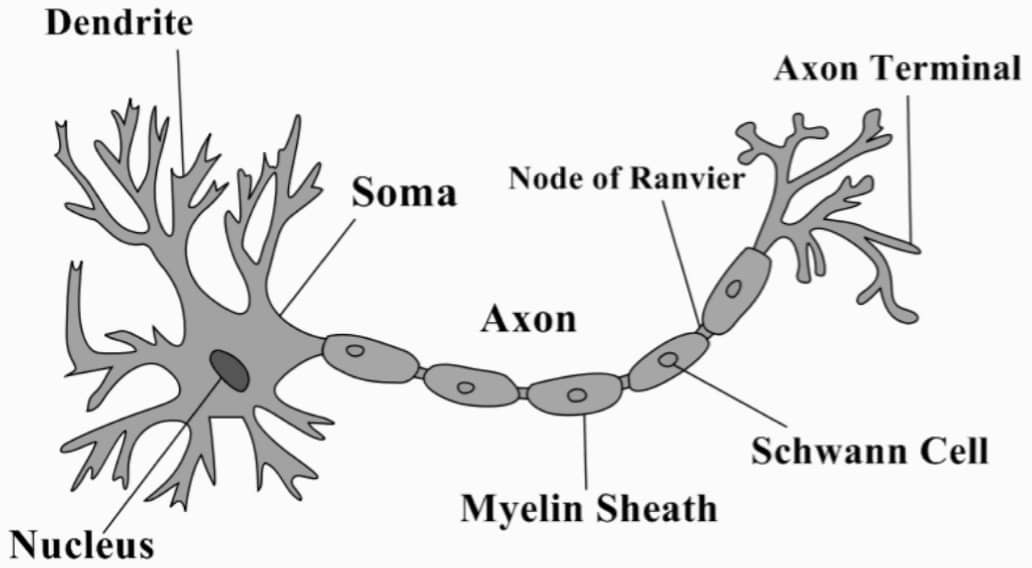
Q23. Draw a well labelled diagram of a neuron.
Ans –
Q24. Name the mechanism by which hormone secreted in precise quantity. Give an example.
Ans – The mechanism by which hormones are secreted in precise quantity is called Feedback Mechanism. An example of this is the regulation of insulin secretion by the pancreas. When blood glucose levels rise, the pancreas secretes insulin to lower glucose levels. As glucose levels decrease, insulin secretion decreases as well. This feedback loop helps maintain stable blood glucose levels.
Q25. What are analogous organs? Write an example.
Ans – Organs in different species which have the different structural design and origin but similar functions.
Example : wings of birds and wings of insects
Q26. Write about various contraceptive methods.
Ans – A method or device used to prevent pregnancy is known as contraception. It is also known as birth control, anticonception, or fertility control.
Methods of contraception :
(i) Natural methods : Coitus interrupts or withdrawal
(ii) Barriers : use Condoms
(iii) Intra-uterine devices (IUDs) : Non-medicated IUDs and copper releasing IUDs,
(iv) Oral contraceptives : Oral administration of progestogens or progestogen–estrogen combos in tiny dosages as a tablet or pill.
(v) Injectables : Females can also use progestogens alone or in combination with estrogen as injections
(vi) Implants : Females can also use progestogens alone or in combination with estrogen as implants on skin.
(vii) Surgical methods : Vasectomy in males and Tubectomy in females.
Q27.(a) Draw a well labelled diagram of stomata. Explain how does the stomatal pore open and close ?
Ans – Stomata are small pores which are present on the surface of the epidermal cells of the plant. It consists of two guard cells, a pore between them and subsidiary cells. The two guard cells have thick walls and are filled with a large amount of cytoplasm.
The guard cells regulate the opening and closing of the stomatal pore. When the guard cells absorb water, they swell up and the stomatal pore opens. This is due to the increase in turgor pressure in the guard cells. On the other hand, when the guard cells lose water, they become flaccid and the stomatal pore closes. This is due to the decrease in turgor pressure in the guard cells.
The opening and closing of the stomatal pore is regulated by the guard cells. The guard cells absorb and lose water depending on the environmental conditions, thus affecting the size of the stomatal pore.
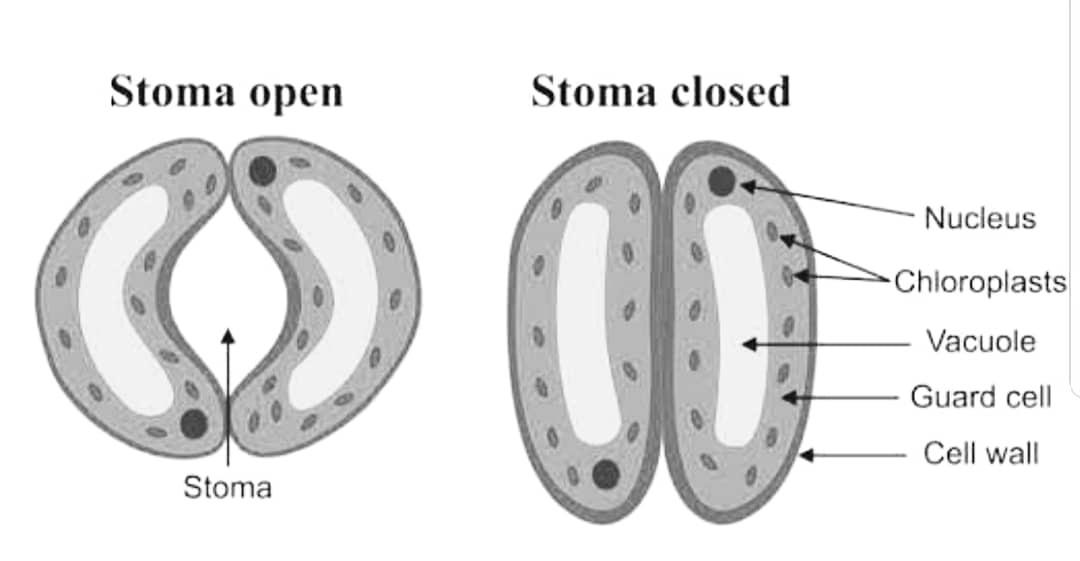
(b) What are the functions of transpiration ?
Ans – Transpiration helps in the absorption and upward movement of water in plants. It helps in the movement of dissolved minerals from the root to leaves. It helps in regulating the temperature of plant or cooling of the plant.
OR
Write down the functions of various digestive enzymes to digest the food in alimentary canal.
Ans – Amylase : Breaks down carbohydrates into simple sugars.
Protease : Breaks down proteins into amino acids.
Lipase : Breaks down fats into fatty acids and glycerol.
Nuclease : Breaks down nucleic acids into nucleotides.
Sucrase : Breaks down sucrose into simple sugars.
Maltase : Breaks down maltose into simple sugars.
Lactase : Breaks down lactose into simple sugars.
SET–C
(Physics)
Q1. Colour of the sun at sunrise is red due to :
(A) Scattering
(B) Dispersion
(C) Refraction
(D) Reflection
Ans – (A) Scattering
Q2. Which of the following gas is greenhouse gas ?
(A) Carbon dioxide
(B) Methane
(C) Oxygen
(D) None of these
Ans – (A) Carbon dioxide
Q3. SI unit of electric resistance is :
(A) Volt
(B) Ampere
(C) Ohm
(D) Coulomb
Ans – (C) Ohm
Q4. Meaning of second R in five R’s is :
(A) Reduce
(B) Recycle
(C) Reuse
(D) All of the above
Ans – (A) Reduce
Order : (i)Refuse, (ii)Reduce, (iii)Reuse, (iv)Repurpose, (v)Recycle
Q5. What are the disadvantages of fossil fuel? Explain in brief.
Ans – Pollution is a major disadvantage of fossil fuels. When burned fossil fuel, they release carbon dioxide which cause greenhouse effect. The fossil fuels are non-renewable sources of energy.
Q6. An electric bulb is connected to a 220 V generator. The current is 0.50A. What is the power of the bulb ?
Ans : Power (P) = V × I = 220 × 0.50 = 110 Watts
Q7. Why does the sky appear dark instead of blue to an astronaut ?
Ans – To an astronaut, the sky looks dark and black instead of blue because there is no atmosphere containing air in the outer space to scatter sunlight. So, there is no scattered light to reach our eyes in outer space, therefore the sky looks dark and black there.
Q8. What is an electric motor ? Draw its labelled diagram and write its principle.
Ans : An electric motor is a device that converts electrical energy into mechanical energy.
Principle – It works on the principle of electromagnetic induction, where a magnetic field is created by passing an electric current through a coil of wire, which then interacts with a permanent magnet to produce rotational motion.
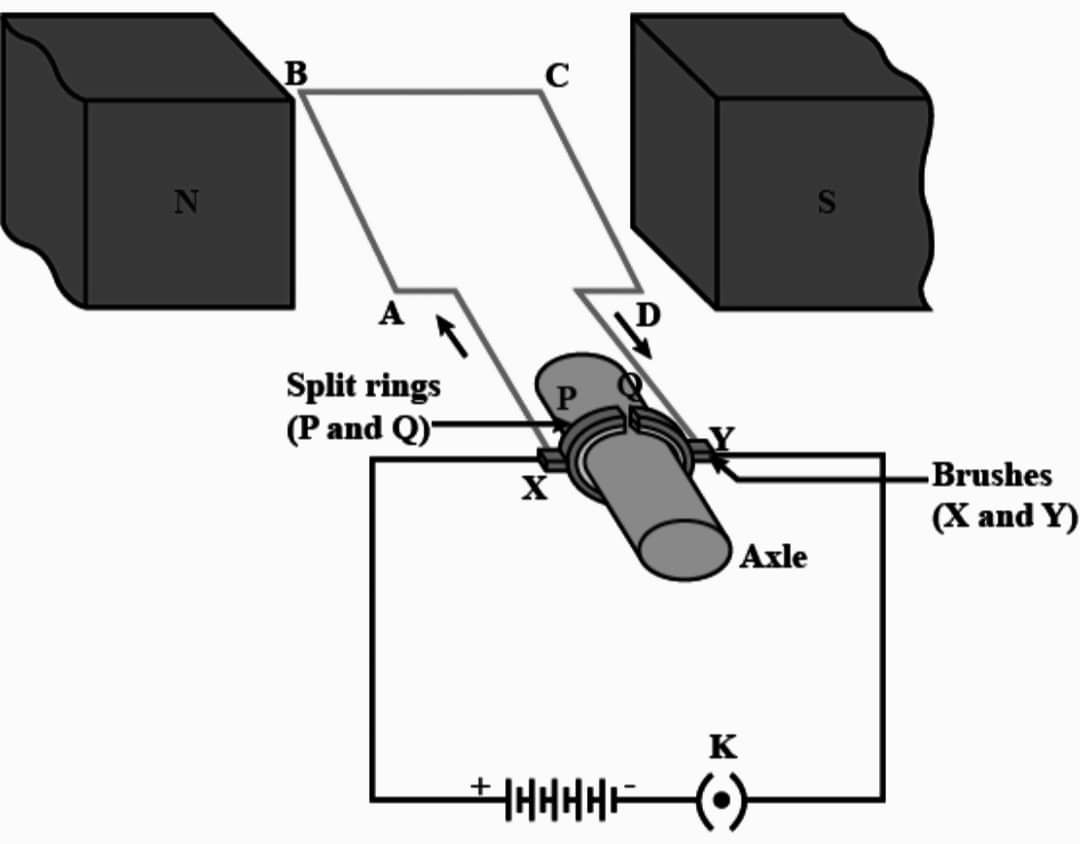
Working – Current in the coil ABCD enters from the source battery through conducting brush X and flow back to the battery through brush Y. Notice that the current in the arm AB of the coil flows from A to B. In arm CD it flows from C to D that is opposite to the direction of current through arm AB on applying Fleming’s left hand rule for the direction of force on a current-carrying conductor in a magnetic field. We find that the force acting on arm AB pushes it downwards while the force acting on arm CD pushes it upwards. Thus the coil and the Axle O, mounted free to turn about an axis, rotate anti-clockwise at half rotation. Q makes contact with the brush X and P with brush Y. Therefore the current in the coil gets reversed and flows along the path DCBA. The reversal of current also reverses the direction of force acting on the two arms AB and CD. Thus the arm AB of the coil that was earlier pushed down, is now pushed up and the arm CD previously pushed up is pushed down. There is a continuous rotation of the coil and to the axle.
Q9.(a) Write the laws of reflection of light.
Ans – The incident ray, the reflected ray and the normal at the point of incidence, all lie in the same plane.
The angle of incidence is equal to the angle of reflection. i.e. ∠i = ∠r

(b) An object is placed between the centre of curvature (C) and focus (F) of a concave mirror. Draw the ray diagram to depict the position, size and nature of image formed.
Ans – The image is real, inverted and larger than object.
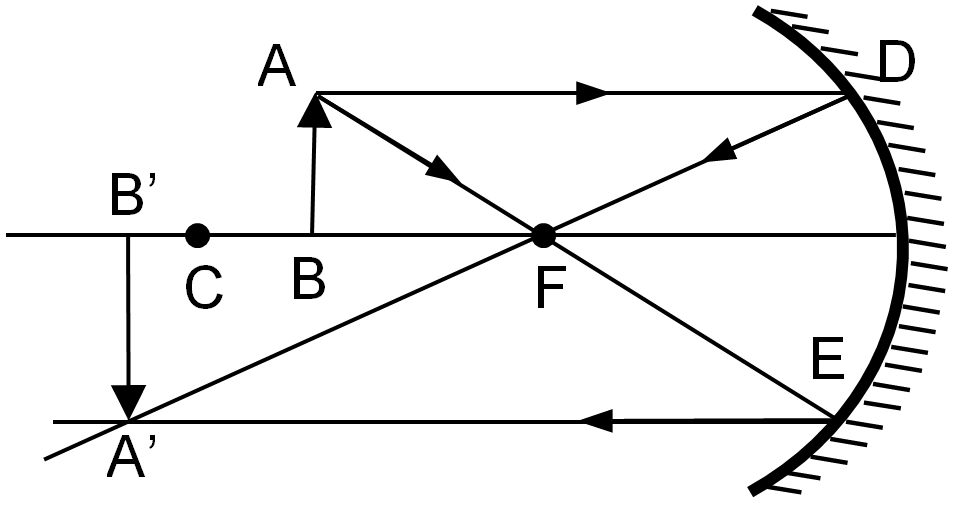
(c) Find the focal length of a lens of power -2.0 D. What type of lens is this ?
Ans : Focal length (F) = 1/P = 1/(-2) = -0.5 m
It is a concave lens due to negative focal length.
OR
(a) Write the laws of refraction of light.
Ans – The incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
The ratio of the sine of the angle of incidence (i) to the sine of the angle of refraction (r) is constant for the pair of given medium. i.e sini/sinr = constant

(b) Draw a ray diagram to show the image formed by a convex lens when an object is placed beyond 2F1.
Ans – The image is real, inverted and smaller than object.
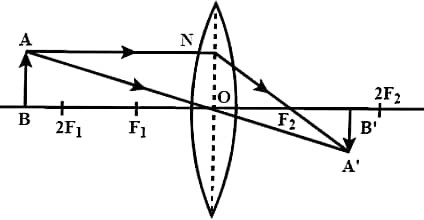
(c) Why do we prefer a convex mirror as a rear view mirror in vehicles ?
Ans – Because they give an erect, virtual, full size diminished image of distant objects with a wider field of view. Thus, convex mirrors enable the driver to view much larger area.
(Chemistry)
Q10. In rice, potatoes and bread what is mainly found ?
(A) Proteins
(B) Carbohydrates
(C) Fats
(D) Vitamins
Ans – (B) Carbohydrates
Q11. Which salt is used in the removal of permanent hardness of water ?
(A) Sodium Hydroxide
(B) Sodium Carbonate
(C) Sodium Chloride
(D) Calcium Chloride
Ans – (B) Sodium Carbonate
It is also known as Washing soda.
Q12. How many electrons are in outermost shell of Group-17? (Modern Periodic Table)
(A) 8
(B) 7
(C) 10
(D) 2
Ans – (B) 7
Q13. What is the colour of Ferrous sulphate crystals? How does this colour change after heating ?
Ans – The colour of ferrous sulphate crystals (FeSO4.7H2O) is light Green. On heating, ferrous sulphate crystals lose water and anhydrous ferrous sulphate (FeSO4) is formed. So their colour changes from light green to white.
Q14. Four solution A, B, C and D when tested with a universal indicator, showed pH as 8, 6, 13 and 2 respectively. Which solution is neutral, strongly alkaline, strongly acidic and weakly acidic or weakly alkaline ?
Ans – The pH scale is a measure of acidity or alkalinity of a solution and ranges from 0 to 14. A pH of 7 is considered neutral, below 7 is acidic and above 7 is alkaline. The scale is logarithmic, meaning that each whole number increase or decrease represents a ten-fold increase or decrease in acidity or alkalinity.
Solution A(pH=8) is Weakly Alkaline
Solution B(pH=6) is Weakly Acidic
Solution C(pH=13) is Strongly Alkaline
Solution D(pH=2) is Strongly Acidic
Q15. Nitrogen (Atomic No. 7) and Silicon (Atomic No. 14) belong to group 15 in periodic table. Give electronic configuration and which is more electronegative, why ?
Ans – Electronic configuration of Nitrogen is (2, 5) and Silicon is (2, 8, 4).
Nitrogen is more electronegative than Silicon.
Q16.(i) What is reactivity series? Describe with example.
Ans – Reactivity series is a list of metals arranged in decreasing(high to low) order of their reactivity. Most reactive metals are at the top while the least reactive metals at the bottom.
Example : Therefore, the highly reactive metal (Potassium) is at the top of this series, while the least reactive metal (Platinum) is placed at the bottom.
(ii) Write the equations for the reactions of :
(a) iron with steam
Ans : 3Fe + 4H2O → Fe3O4 + 4H2
(b) Calcium with steam
Ans : Ca + 2H2O → Ca(OH)2 + H2
Q17. Complete the following chemical reactions :
(i) CH3COOH + NaHCO3 →
Ans : CH3COONa + CO2 + H2O
(ii) Na + C2H5OH →
Ans : C2H5ONa + ½H2
(iii) C2H5OH + Hot Conc.H2SO4 →
Ans : CH2=CH2 (ethene)
(b)(i) Make a structure of Benzene.
Ans –

(ii) Write a short note on Micelles.
Ans – The unique orientation of soap/detergent molecules inside the water which prevents the contact of the hydrophobic (water fearing) part of the molecule with water is known as a micelle.
OR
(a) Write the names of following compounds :
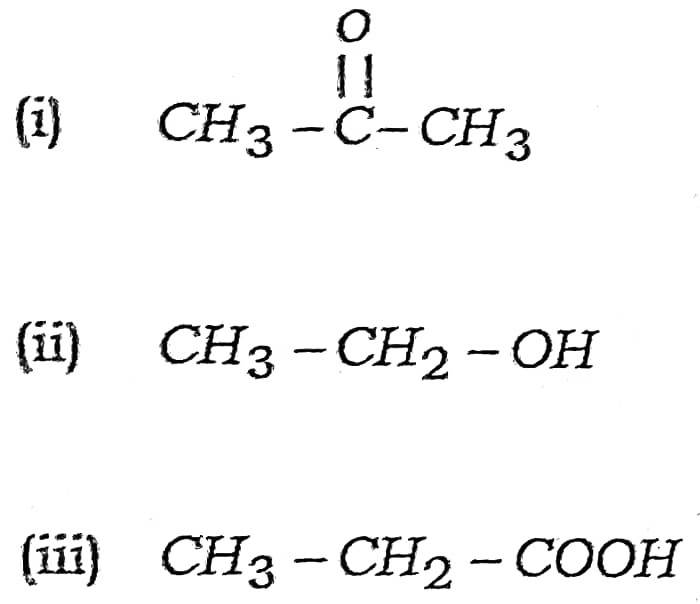
Ans –
(i) Propanone
(ii) Ethanol
(iii) Ethanoic acid
(b)(i) Explain addition reaction with example.
Ans – A chemical reaction where two or more reactants combine to form a single product is called an addition reaction.
Example : 3Fe + 2O2 → Fe3O4
(ii) Make a structure of Benzene.
Ans –

(Biology)
Q18. Which hormone inhibits the growth in plants ?
(A) Auxin
(B) Cytokinins
(C) Gibberellins
(D) Abscisc acid
Ans – (C) Abscisc acid
Q19. Which is a recessive traits in pea plant ?
(A) Tall plant
(B) Round seed
(C) Both (A) and (B)
(D) White flower
Ans – (D) White flower
Q20. In Leishmania reproduction take place by :
(A) Binary fission
(B) Multiple fission
(C) Budding
(D) Spore formation
Ans – (A) Binary fission
Q21. Gregor Mendel belonged to which country ?
Ans – Austria
Q22. What is the role of the brain in reflex action ?
Ans – Brain do not play any role in reflex action.
Q23. What are the various parts of peripheral nervous system? Write their functions.
Ans – The peripheral nervous system (PNS) is made up of two parts :
Somatic Nervous System – The somatic nervous system is responsible for the voluntary control of body movement and sensation.
Autonomic Nervous System – The autonomic nervous system is responsible for controlling the involuntary functions of the body, such as heart rate, digestion, and breathing.
Q24. What are the advantages and disadvantages of ozone ?
Ans : Advantage of Ozone – The ozone layer surrounding the earth’s atmosphere acts as a shield by absorbing the harmful ultraviolet radiations of the sunlight to protect the living organisms from the harmful effects of UV rays.
Disadvantage of Ozone – On ground level ozone is poisonous gas.
Q25. How do we know how old the fossils are ?
Ans : Fossils can be dated by studying the layers of rock in which they are found. Scientists use a variety of methods :
Radiometric Dating – In this compares the ratios of certain elements in a sample to determine its age.
Relative Dating – In this orders fossils based on the age of the surrounding rocks, or the stratigraphic record, which uses fossils to help place rock layers in chronological order.
Q26. What happens when the egg is not fertilized ?
Ans – When the egg is not fertilized then Menstruation occurs.
Q27.(a) Draw a labelled diagram of structure of a nephron.
Ans –
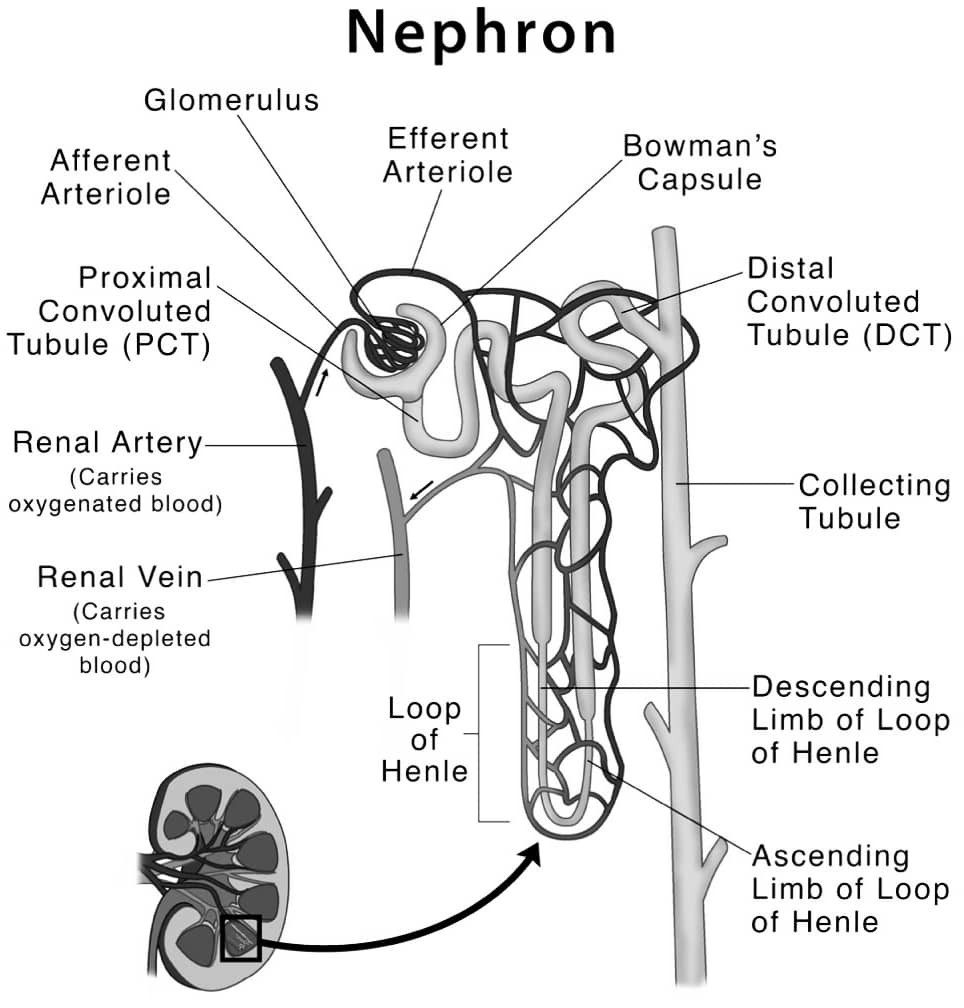
(b) How is urine formed in human beings ?
Ans – Urine is formed in the kidneys through a process known as urine formation or urine production. The process involves three main steps :
Filtration – Blood from the renal artery enters the glomerulus, a network of tiny blood vessels in the kidney. The pressure in the glomerulus forces water, dissolved substances, and small molecules like urea, uric acid, and creatinine out of the blood and into the Bowman’s capsule, a small sac-like structure surrounding the glomerulus.
Reabsorption – The filtrate then passes through a series of tubules in the kidney, where most of the water, electrolytes, and nutrients that the body needs are reabsorbed back into the bloodstream. This is an important step to maintain the balance of fluids and electrolytes in the body.
Secretion – The remaining fluid, which is now urine, is collected in the collecting ducts and transported down to the bladder through the ureters. Along the way, the kidneys can also secrete additional substances into the urine, such as excess potassium or hydrogen ions, to further regulate the body’s fluid balance and pH level.
Once the urine reaches the bladder, it is stored until it is eliminated from the body through the urethra during urination.
OR
(a) Describe mechanism of respiration in human beings.
Ans – Respiration in human beings involves the process of exchanging gases between the body and the environment. It is a complex process that involves several organs and structures, including the lungs, diaphragm, and respiratory muscles.
The process of respiration begins with inhaling air through the nose or mouth. The air then travels down the trachea, which branches out into the two bronchi, each leading to one of the lungs. Inside the lungs, the bronchi divide into smaller and smaller tubes called bronchioles, which eventually lead to tiny air sacs called alveoli.
The alveoli are surrounded by capillaries, which are tiny blood vessels that transport oxygen and carbon dioxide to and from the body’s cells. When oxygen enters the alveoli, it diffuses through the walls of the air sacs and capillaries and into the bloodstream. At the same time, carbon dioxide, which is a waste product of cellular metabolism, diffuses from the capillaries into the alveoli.
The process of breathing is controlled by the respiratory center in the brainstem. When the body needs more oxygen, the respiratory center sends signals to the respiratory muscles, including the diaphragm and intercostal muscles, to contract. This causes the volume of the chest cavity to increase, which in turn decreases the pressure inside the lungs. This decrease in pressure causes air to rush into the lungs, filling the alveoli with fresh air.
When the body needs to exhale carbon dioxide, the respiratory center sends signals to the respiratory muscles to relax, causing the volume of the chest cavity to decrease. This increase in pressure forces air out of the lungs, expelling the carbon dioxide from the body.
Overall, the process of respiration is essential for sustaining life, providing the body with oxygen and removing carbon dioxide, which is a waste product of cellular metabolism.
(b)(i) What is Lymph ?
Ans – Lymph is a clear, watery fluid that circulates throughout the body. It is part of the lymphatic system.
(ii) How does it form ?
Ans – Lymph is produced when the small blood vessels in the body (capillaries) filter fluid from the tissue spaces back into the bloodstream. The filtered fluid then moves through the lymphatic system and is eventually collected in the lymph nodes.
(iii) What are its function ?
Ans – The main function of lymph is to transport immune cells throughout the body and help fight infection and disease. It also helps to remove toxins, debris, and other unwanted substances from the body. Additionally, lymph helps to maintain fluid balance and delivers essential nutrients to the cells.
SET–D
(Physics)
Q1. The human eye forms the image of an object at its :
(A) Cornea
(B) Retina
(C) iris
(D) Pupil
Ans – (B) Retina
Q2. Which of the following community of Rajasthan has a religious tenet of conservation of forest and wildlife ?
(A) Bishnoi
(B) Jain
(C) Agarwal
(D) Brahmin
Ans – (A) Bishnoi
Q3. SI unit of potential difference is :
(A) Volt
(B) Joule
(C) Watt
(D) Coulomb
Ans – (A) Volt
Q4. Ganga action plan was started in :
(A) 1945
(B) 1965
(C) 1985
(D) 1999
Ans – (C) 1985
Q5. What are the advantages and disadvantages of using a solar cooker ?
Ans : Advantages – There is no cost of fuel and the cost of solar cooker is very low.
Disadvantages – It is slow and can not be used in rainy or cloudy days.
Q6. Why does the sun appear reddish early in the morning ?
Ans – During sunrise, the light rays coming from the Sun, is at the horizon and thus light has to travel a greater distance in the earth’s atmosphere before reaching our eyes. In this journey, the shorter wavelengths of lights are scattered out and only longer wavelengths are able to reach our eyes. Since blue colour has a shorter wavelength and red colour has a longer wavelength, the red colour is able to reach our eyes after the atmospheric scattering of light. Therefore, the Sun appears reddish early in the morning.
Q7. An electric motor takes 5 A from a 220 V line. Determine the power of the motor.
Ans : Power (P) = V × I = 220 × 5 = 1100 Watts
Q8. What is an electric generator? Draw its labelled diagram and write its principle.
Ans : An electric generator is a device that converts mechanical energy into electrical energy.
Principle – It works on the principle of electromagnetic induction, where a magnetic field is rotated inside a coil of wire, which then induces an electric current in the wire.
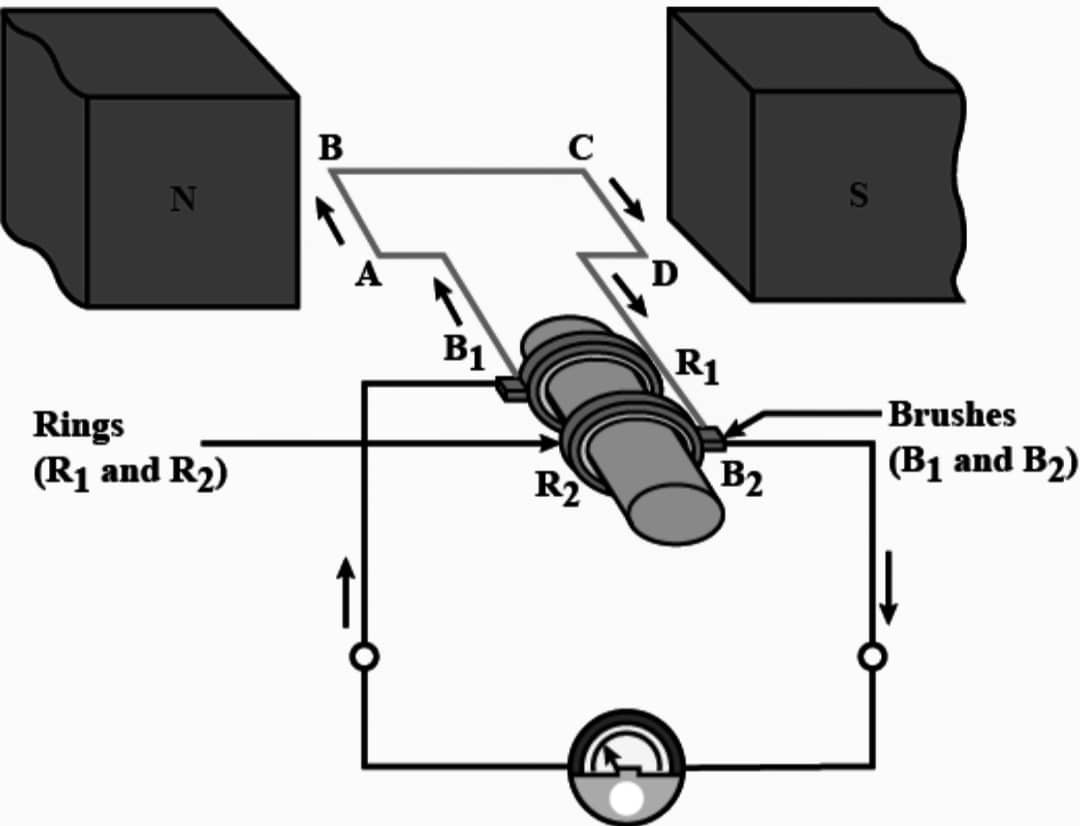
Working – When the axle attached to the two rings is rotated such that the arm AB moves up (and the arm CD moves down) in the magnetic field produced by the permanent magnet. Let us say the coil ABCD is rotated clockwise in the arrangement. By applying Fleming’s right hand rule, the induced currents are set up in these arms along the directions AB and CD. Thus an induced current flows in the direction ABCD. If there are larger number of turns in the coil, the current generated in each turn adds up to give a large current through the coil. This means that the current in the external circuit flows from B2 to B1. After half a rotation, arm CD starts moving up and AB moving down. As a result, the directions of the induced currents in both the arms changes, giving rise to the net induced current in the direction DCBA. The current in the external circuit now flows from B1 to B2. Thus after every half rotation the polarity of the current in the respective arms changes.
There are two brushes and in the electric generator, one brush is at all times in contact with the arm moving up in the field, while the other is in contact with the arm moving down. Because of these Brushes unidirectional current is produced. Function of brushes is to transfer the current from coil to load connected in the circuit of the electric generator.
Q9.(a) Write the laws of reflection of light.
Ans – The incident ray, the reflected ray and the normal at the point of incidence, all lie in the same plane.
The angle of incidence is equal to the angle of reflection. i.e. ∠i = ∠r

(b) An object is placed between the main focus (F) and the optical centre (O) of a convex lens. Draw a ray diagram showing the position, size and nature of the image formed.
Ans – The image is virtual, erect and larger than object.
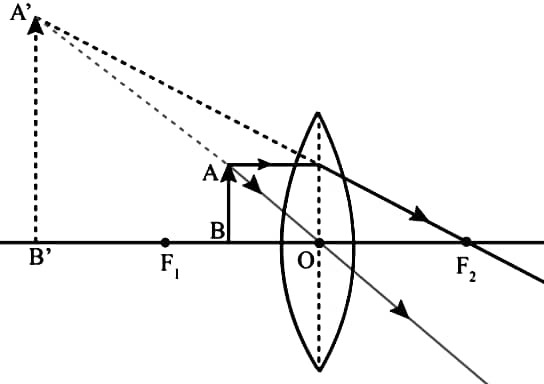
(c) What is the power of a lens? Define 1 Dioptre (1D) power of a lens.
Ans – Power of a lens is its ability to converge or diverge the rays of light falling on it. Power of a lens is equal to reciprocal of the focal length of the lens. SI unit of power is dioptre (D).
1 Dioptre is the power of the lens whose focal length is 1 m.
OR
(a) Write the laws of refraction of light.
Ans – The incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
The ratio of the sine of the angle of incidence (i) to the sine of the angle of refraction (r) is constant for the pair of given medium. i.e sini/sinr = constant

(b) An object is placed between the centre of curvature (C) and principal focus (F) of a concave mirror. Draw the ray diagram to depict the position, size and nature of image formed.
Ans – The image is real, inverted and larger than object.

(c) Find the focal length of a lens of power -2.0 D. What type of lens is this ?
Ans : Focal Length (F) = 1/P = 1/(-2) = -0.5 m
It is a concave lens due to negative focal length.
(Chemistry)
Q10. Nitrogen gas prevent to chips in bags from a process of :
(A) Reduced
(B) Hydrogenation
(C) Displacement
(D) Oxidised
Ans – (D) Oxidised
Q11. Which following solution is acidic in nature ?
(A) Lime juice
(B) Human blood
(C) Slaked lime
(D) Antacid
Ans – (A) Lime juice
Q12. What is radius of hydrogen atom ?
(A) 20 pm
(B) 37 pm
(C) 42 pm
(D) 50 pm
Ans – (B) 37 pm
Q13. What do you observe when a piece of iron is dropped in copper sulphate solution? Give the chemical reaction.
Ans – When a few pieces of iron are dropped into a blue coloured copper sulphate solution, the blue colour of the solution fades and eventually turns into Green. This is a dispacement reaction.
CuSO4 + Fe → FeSO4 + Cu
Q14. What is pH scale in chemistry? Explain.
Ans – A pH scale is measure acidity or basicity of a substance or solution. pH is measured on a scale of 0 to 14. On this scale, a pH value of 7 is neutral, which means it is neither acidic nor basic. A pH value of less than 7 means it is acidic, and a pH value of more than 7 means it is basic.
Q15. An atom has electronic configuration 2, 8, 8, 2 :
(a) What is the atomic number of this element ?
Ans – Atomic number is 20 (2+8+8+2) of Calcium.
(b) Give a compound formula for this element.
Ans : CaCO3 (Calcium Carbonate)
Q16.(a) What is Calcination? Give an example.
Ans – The process of heating the ore strongly below its melting point and thus converting the ores into oxides is called calcination. It is usually done for hydroxide and carbonate ores.
Example : MgCO3 + ∆ → MgO + CO2
Here, magnesium carbonate is heated in the absence of air to gain magnesium oxide as the product.
(b) Write a short note on electrolytic refining of Copper.
Ans – Electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. In this method, the anode is made from impure copper, while the cathode is made from pure copper. The electrolyte is made from acidified copper sulphate solution. When electricity is passed, the impure copper from the anode gets dissolved in the electrolyte, while pure copper gets deposited on the cathode. In this way, pure copper is obtained from the cathode.
Q17.(a) Write the names of following compounds :
(i) CH3–CH2–CH2–CH3
Ans : Butane
(ii) CH3–OH
Ans : Methanol
(iii) CH3–CH2–CI
Ans : Chloroethane
(b) Explain alkane, alkene and alkyne with suitable example.
Ans – Alkanes (CnH2n+2) have only single bonds between carbon atoms. Alkanes are called saturated hydrocarbons. e.g. Methane (CH4)
Alkenes (CnH2n) have at least one carbon-carbon double bond. Alkenes are called unsaturated hydrocarbons. e.g. Butene (C4H8)
Alkynes (CnH2n-2) have one or more carbon-carbon triple bonds. Alkynes are also called unsaturated hydrocarbons. e.g. Ethyne (C2H2)
OR
(a) Complete the following chemical reactions :
(i) CH3–CH2–OH + O2 →
Ans : 2CO2 + 3H2O + Heat and Light
(ii) CH3CH2OH + Alkaline KMnO4 hot →
Ans : CH3–COOH
(iii) CH3COOC2H5 + NaOH →
Ans. CH3COONa + C2H5OH
(b) Give difference between saturated hydrocarbon and unsaturated hydrocarbon with two suitable example.
Ans. Saturated hydrocarbon – The simplest hydrocarbons in which carbon-carbon atoms and carbon-hydrogen atoms are held together by single bonds are known as saturated hydrocarbon . e.g. CH3–CH3
Unsaturated Hydrocarbons – The hydrocarbons in which Carbon-Carbon atoms and Carbon-Hydrogen atoms are held together by the double or triple bond are known as unsaturated hydrocarbons. e.g. CH2=CH2 , HC≡CH
(Biology)
Q18. Where does fertilization take place in human being ?
(A) Ovary
(B) Fallopian tube
(C) Uterus
(D) Vagina
Ans – (B) Fallopian tube
Q19. Which vegetable is developed for sterile flowers from cultivated wild cabbage by selection ?
(A) Cabbage
(B) Cauliflower
(C) Broccoli
(D) Kale
Ans – (B) Cauliflower
Q20. Which is an artificial ecosystem ?
(A) Forest
(B) Pond
(C) Lake
(D) Garden
Ans – (D) Garden
Q21. What is meant by characteristics ?
Ans – Characteristics are the distinguishing features of an organism. These are the details of the behaviour or appearance of an organism.
Q22. Which organisms act as decomposers ?
Ans – Bacteria, fungi and protozoa
Q23. What are the limitations to the use of electrical impulses ?
Ans – The impulses can move only in one direction. This is because the nerves are structured to allow unidirectional flow.
The electrical impulses are quite short acting. The message is only sent in the presence of the stimulus.
Q24. Which gland secrete the growth hormone? What happens due to deficiency and excess of this hormone ?
Ans – The pituitary gland is secrete the growth hormone. Excess secretion of this hormone leads to gigantism and less secretion of this hormone leads to dwarfism.
Q25. What are homologous organs? Give an example.
Ans – The organs found in different organisms which have a common origin and same basic structure but differ in the functions they carry out are called homologous organs.
Examples of homologous organs include the forelimbs of a man, the forelimbs of a cat and flippers of a whale.
Q26. What are the changes seen in girls and boys at time of puberty ?
Ans : Puberty is a time of significant physical and emotional changes that occur as a child’s body matures into an adult. While both girls and boys experience many of the same changes during puberty, there are also some differences between the sexes.
In girls – puberty typically begins between the ages of 8 and 13 years old. Some of the most noticeable changes during puberty in girls include the development of breasts, the growth of pubic and underarm hair, the beginning of menstruation, and an increase in height and weight. Girls may also experience mood swings, acne, and increased body odor.
In boys – puberty typically begins between the ages of 9 and 14 years old. Some of the most noticeable changes during puberty in boys include the growth of testicles and penis, the growth of pubic and underarm hair, a deepening of the voice, an increase in height and weight, and the development of facial hair. Boys may also experience mood swings, acne, and increased body odor.
It’s important to note that each child’s experience of puberty is unique, and the timing and progression of puberty can vary widely. If you have concerns about your child’s development or any aspect of their health, it’s important to speak with a healthcare professional.
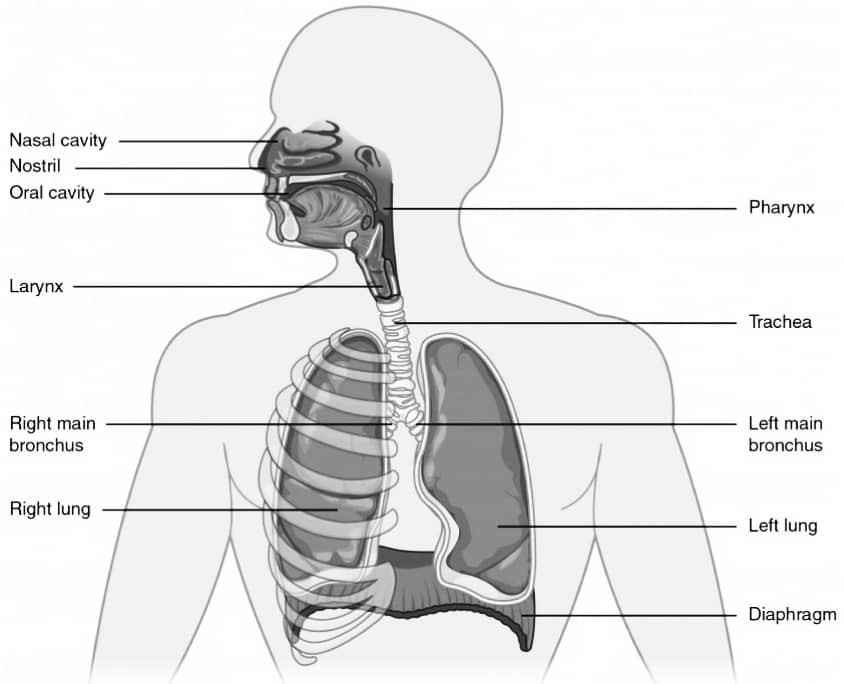
Q27. Explain the structure of human respiratory system with the help of well labelled diagram.
Ans : The human respiratory system consists of the following structures :
Nasal cavity – It is the first structure that air passes through. It is lined with tiny hairs called cilia and mucus-producing cells that help to filter and moisten the air.
Pharynx – It is a muscular tube that connects the nasal cavity to the larynx and esophagus. It serves as a common passage for both air and food.
Larynx – It is commonly known as the voice box and contains the vocal cords. It helps to regulate the flow of air into the trachea and prevents food from entering the lungs.
Trachea – It is a tube-like structure that extends from the larynx to the bronchi. It is lined with cilia and mucus-producing cells that help to filter and moisten the air.
Bronchi – They are two branches of the trachea that lead to the left and right lungs. They further divide into smaller tubes called bronchioles.
Lungs – They are the main organs of respiration and are divided into lobes. The right lung has three lobes, while the left lung has two lobes.
Alveoli – They are tiny air sacs located at the end of the bronchioles. They are responsible for gas exchange between the lungs and blood vessels.
OR
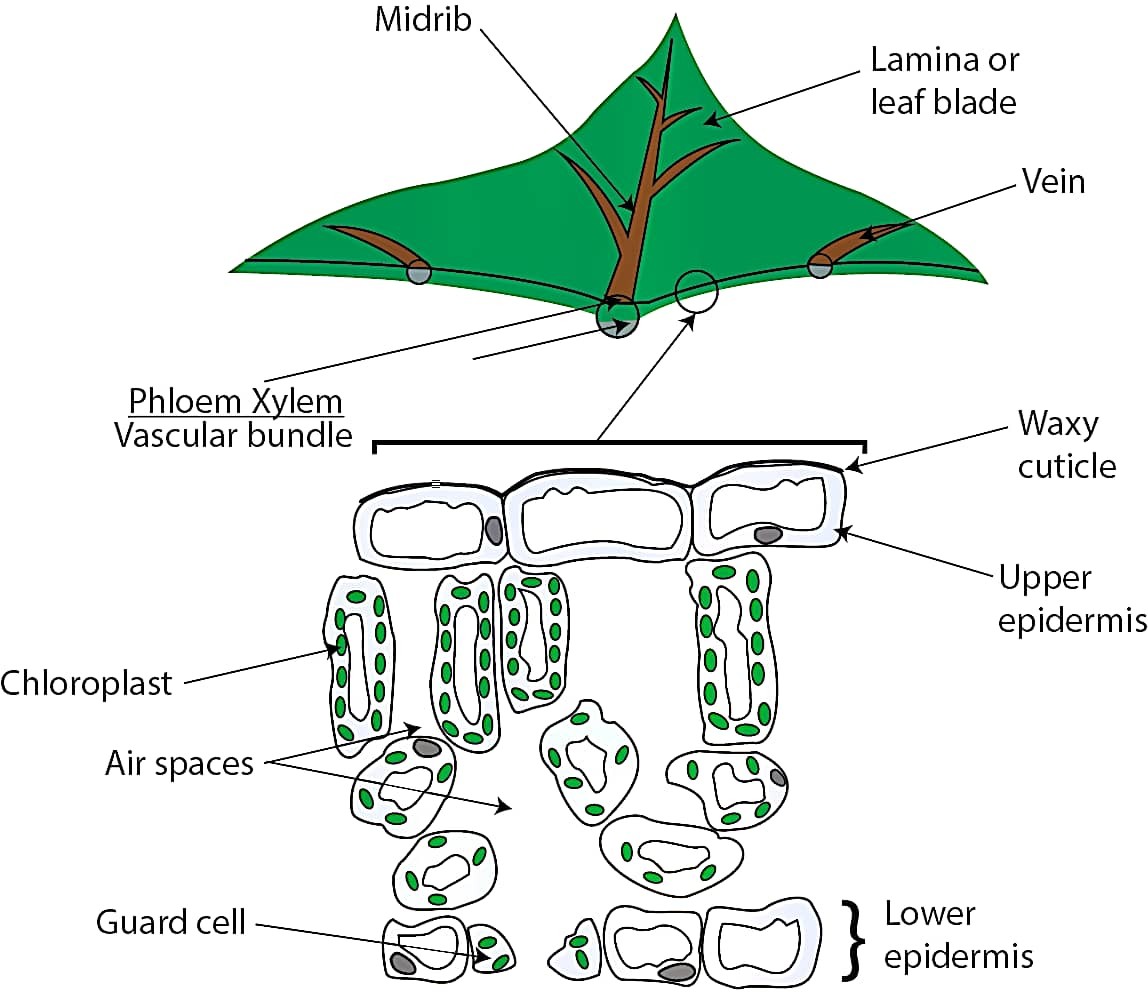
(a) Draw a well labelled diagram of cross section of a leaf.
Ans –
(b) Write the various events which occur during photosynthesis.
Ans – The events that occur during the process of photosynthesis are :
(i) Absorption of light energy by chlorophyll.
(ii) Conversion of light energy to chemical energy and splitting of water molecules into hydrogen and oxygen.
(iii) Reduction of carbon dioxide to carbohydrates.
