Haryana Board (HBSE) Class 10 Science Pre Board Question Paper 2024 Answer Key. Haryana Board Class 10th Pre Board Question Paper PDF Download 2024. HBSE Class 10 Science Solved Question Paper 2024. Haryana Board Class 10th Pre Board Question Paper Science 2024. HBSE Class 10th Science Pre Board Question Paper Solution 2024. HBSE Science Pre Board Question Paper 2024 Class 10.
HBSE Class 10 Science Pre-Board Question Paper 2024 Answer Key
SECTION – A (Physics)
1. A full length image of a distant tall building can definitely be seen by using : (1 Mark)
(A) a concave mirror
(B) a convex mirror
(C) a plane mirror
(D) both concave as well as plane mirror
Answer – (B) a convex mirror
2. A positively-charged particle (alpha-particle) projected towards west is deflected towards north by a magnetic field. The direction of magnetic field is : (1 Mark)
(A) towards south
(B) towards east
(C) downward
(D) upward
Answer – (D) upward
3. Copper and aluminium are used for electric transmission lines because both posses ……………. resistivity. (1 Mark)
Answer – Low
4. Define power of accommodation. (1 Mark)
Answer – Power of accommodation is the ability of the eye lens to focus near and far objects clearly on the retina by adjusting its focal length. In this process ciliary muscles help in changing the shape of lens by compressing and elongating it.
5. Assertion (A) : White light is dispersed into its seven-colour components by a prism. (1 Mark)
Reason (R) : Different colours of light bend through different angles with respect to the incident ray as they pass through a prism.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer – (a) Both A and R are true and R is the correct explanation of A.
6. Show how would you join three resistors each of resistance 9 Ω so that the equivalent resistance of the combination is 6 Ω. (2 Marks)
Answer – Two resistors in series and third resistor in parallel.
Rs = R1 + R2 = 9 + 9 = 18 Ω
1/RP = 1/Rs + 1/9 = 1/18 + 1/9 = 3/18 = 1/6
RP = 6 Ω
OR
An electric motor takes 5 A from a 220 V line. Determine the power of the motor and the energy consumed in 2 h. (2 Marks)
Answer – Current (I) = 5 A
Voltage (V) = 220 V
Time (t) = 2 h = 2 × 3600 = 7200 s
Power (P) = V × I = 220 × 5 = 1100 watts
Energy (E) = P × t = 1100 × 7200 = 7.92 × 106 Joules
7. Why do stars twinkle? (2 Marks)
Answer – Stars twinkle due to the atmospheric refraction of starlight.
8. (a) State Ohm’s law. (1 Mark)
Answer – Ohm’s law states that the current (I) flowing through a conductor is directly proportional to the potential difference (V) across the conductor provided all physical conditions and temperature remains constant.
I ∝ V
I = V/R
V = IR
(b) On what factors does the resistance of a conductor depend? (2 Marks)
Answer – The resistance of a conductor depends on the cross sectional area of the conductor, the length of the conductor, temperature, and nature of material. Its SI unit of resistance is Ohm (Ω).
9. (a) Draw magnetic field lines around a bar magnet. List the properties of magnetic field lines. (2 Marks)
Answer –
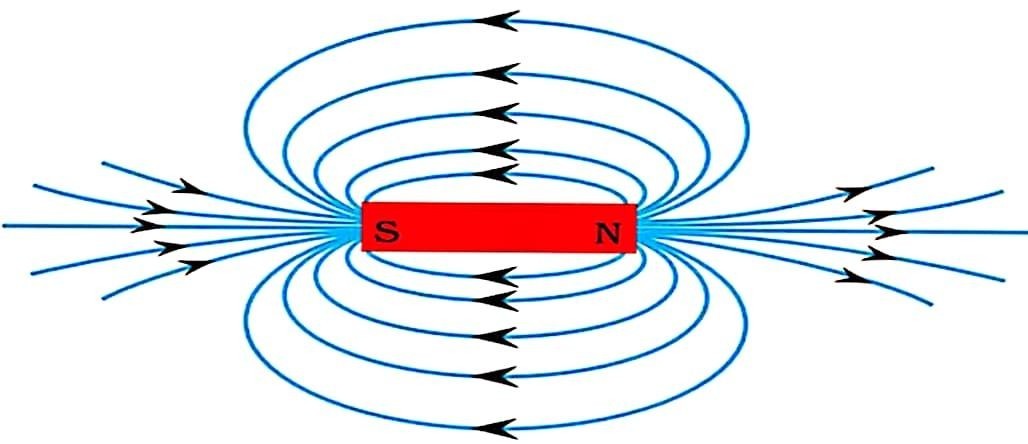
Properties of magnetic field lines :
(i) Two magnetic field lines never intersect each other.
(ii) Magnetic field lines are closed curves.
(iii) Where the magnetic field lines are dense, the magnetic field is strong.
(b) Name two safety measures commonly used in electric circuits and appliances. (1 Mark)
Answer – Electric fuse and earth wire.
OR
(a) What is solenoid? Draw the magnetic field around a current carrying solenoid. (2 Marks)
Answer – Solenoid is a type of electromagnet, the purpose of which is to generate a controlled magnetic field through a coil wound into a tightly packed helix.

(b) When does an electric short circuit occur? (1 Mark)
Answer – The heavy current flow with very low resistance then short circuit occur.
10. (i) The magnification produced by a plane mirror is +1. What does this mean? (1 Mark)
Answer – The magnification produced by a plane mirror is +1 means that the image formed is virtual, erect and of the same size as that of object.
(ii) State the laws of refraction. (2 Marks)
Answer – The incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
The ratio of the sine of the angle of incidence (i) to the sine of the angle of refraction (r) is constant for the pair of given medium. i.e sini/sinr = constant. This is known as Snell’s Law.
(iii) A ray of light travelling in air enters obliquely into water. Does the light ray bend towards the normal or away from the normal? Why? (2 Marks)
Answer – Ray of light travels from air (rarer) to water (denser) then ray of light bends towards the normal.
OR
(i) Define power of lens. What is its Sl unit? If the power of lens is negative what type of lens is it? (2 Marks)
Answer – It is the ability of lens to converse or diverse the ray of light. SI unit of power is Diopter. The lens is concave lens.
(ii) Draw the ray diagram and also state the position, the relative size and the nature of image formed by a convex lens when the object is placed at 2F1 of the lens. (3 Marks)
Answer – The image is real, inverted, same size as the object and Formed at 2F2.

SECTION – B (Chemistry)
11. What is common name of CaOCl2 ? (1 Mark)
(A) Gypsum
(B) Bleaching Powder
(C) Baking Soda
(D) Washing Soda
Answer – (B) Bleaching Powder
12. MnO2 + 4HCI → MnCl2 + 2H2O + Cl2
Identify the substance oxidized in the above equation. (1 Mark)
(A) MnCl2
(B) HCI
(C) H2O
(D) MnO2
Answer – (A) MnCl2
13. ……………. metal can melt while kept on palm. (1 Mark)
Answer – Gallium and Cesium
14. You are given 3 solutions A, B and C with pH value as 2, 5 & 9.5 respectively. Which solution will contain maximum H+ ion? (1 Mark)
Answer – Solution A(2) is highly acidic as it has least pH value.
15. Assertion (A) : Bronze is an alloy of copper and tin. (1 Mark)
Reason (R) : Alloys are heterogeneous mixture of metals with other metals and non-metals.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer – (c) A is true but R is false.
16. Why does the colour of copper sulphate solution change when an iron nail is dipped in it? (2 Marks)
Answer – Iron displace copper because it is more reactive.
CuSO4 + Fe → FeSO4 + Cu
17. What are amphoteric oxides? Give two examples of amphoteric oxides. (2 Marks)
Answer – Those metal oxides which show acidic and basic behaviour are called amphoteric oxides. e.g. Al2O3, ZnO etc.
18. (a) Why should curd and sour substances not be kept in brass and copper vessels? (2 Marks)
Answer – Curd and sour substances contain acids. This acid reacts with brass and copper vessels and make a poisonous substance. This poisonous substance come in contact of food items and make them unfit for consumption.
(b) Give one example of neutralization reaction. (1 Mark)
Answer – A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. e.g.
HCl (acid) + NaOH (base) → NaCl (salt) + H2O
19. (a) Describe the electrolytic refining of copper with a well labelled diagram. (2 Marks)
Answer – In the electrolytic purification of copper, impure copper is made into an anode and a thin line of pure copper is made into a cathode, a copper salt solution is used as an electrolyte.

(b) What is aqua-regia? (1 Mark)
Answer – A mixture of concentrated HCL and HNO3 in the ratio 3 : 1 is called aqua-regia.
OR
(a) Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same? (1 Mark)
Answer – Aluminium is a ductile metal and it has high melting point and good thermal conductivity.
(b) Draw a schematic diagram of the various steps involved in the extraction of metals from ores for metals of medium reactivity and for metals of low reactivity. (2 Marks)
Answer –
20. (a) What is homologous series? Give examples. List any two of its characteristics. (2 Marks)
Answer – A series of organic compounds having same functional group but different in successive member of –CH2 group. e.g. Methanol (CH3OH) and Ethanol (C2H5OH).
Its characteristics : Show similar chemical properties and represented by same general formula.
(b) Write the following reactions : (2 Marks)
(i) Substitution reaction
Answer : CH4 + Cl2 + sunlight → CH3Cl + HCl
(ii) Oxidation reaction
Answer : C2H5OH + 2[O] + Alk. KMNO4 → CH3COOH (ethanoic acid) + H2O
(c) Draw the electron dot structure for cyclopentane. (1 Mark)
Answer –

OR
(a) Explain the mechanism of the cleaning action of soap. (3 Marks)
Answer – Most dirt is oily in nature and as you know, oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap interacts with water while the carbon chain interacts with oil. The soap molecules thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic-end faces outside.

This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water and we can wash our clothes clean.
(b) Write the names of the following compounds : (2 Marks)
(i) CH3–CH2–COOH
Answer : Propanoic acid
(ii) CH3–CHO
Answer : Ethanal
SECTION – C (Biology)
21. Posture and balance of the body is controlled by : (1 Mark)
(A) Pons
(B) Medulla oblongata
(C) Cerebellum
(D) Cerebrum
Answer – (C) Cerebellum
22. A feature of reproduction that is common to Amoeba, Yeast and Spirogyra is that : (1 Mark)
(A) they reproduce asexually
(B) they are all unicellular
(C) they reproduce only sexually
(D) they are all multicellular
Answer – (A) they reproduce asexually
23. The part of the flower which is present in the centre of the flower is ……………. . (1 Mark)
Answer – Pistil (stigma, style, ovary)
24. State the role played by enzyme trypsin in the process of digestion? (1 Mark)
Answer – Trypsin breaks down proteins in amino acid.
25. Assertion (A) : The sex of a child in human beings will be determined by the type of chromosome he/she inherits from the father. (1 Mark)
Reason (R) : A child who inherits ‘X’ chromosome from his father would be a girl (XX), while a child who inherits a ‘Y’ chromosome from the father would be a boy (XY).
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer – (a) Both A and R are true and R is the correct explanation of A.
26. How do Mendel’s experiments show that traits may be dominant or recessive? (2 Marks)
Answer –

OR
How is the sex of the child determined in human beings? (2 Marks)
Answer –

27. Differentiate between biodegradable and non-biodegradable substances. (2 Marks)
Answer : Biodegradable – Those substance which can be decomposed into simpler substance by action of decomposers. e.g. plant waste, paper, cow dung.
• Non-biodegradable – Those substance which cannot be decomposed into simpler substance by action of decomposers. e.g. plastic, glass, DDT.
28. (i) Name the hormones secreted by the following endocrine glands and specify one function of each : (2 Marks)
(a) Thyroid
Answer – Thyoxine hormone (It regulates metabolism of fat, protein, carbohydrate).
(b) Pancreas
Answer – Insulin (It regulates blood glucose level).
(ii) What happens at the synapse between two neurons? (1 Mark)
Answer – A synapse is a gap between two neurons. At the synapse, the electrical signals are converted into chemicals that can easily cross the gap and pass on to the next neuron where they again get converted into electrical signals.
29. Explain female reproductive system with the help of well labelled diagram. (3 Marks)
Answer –
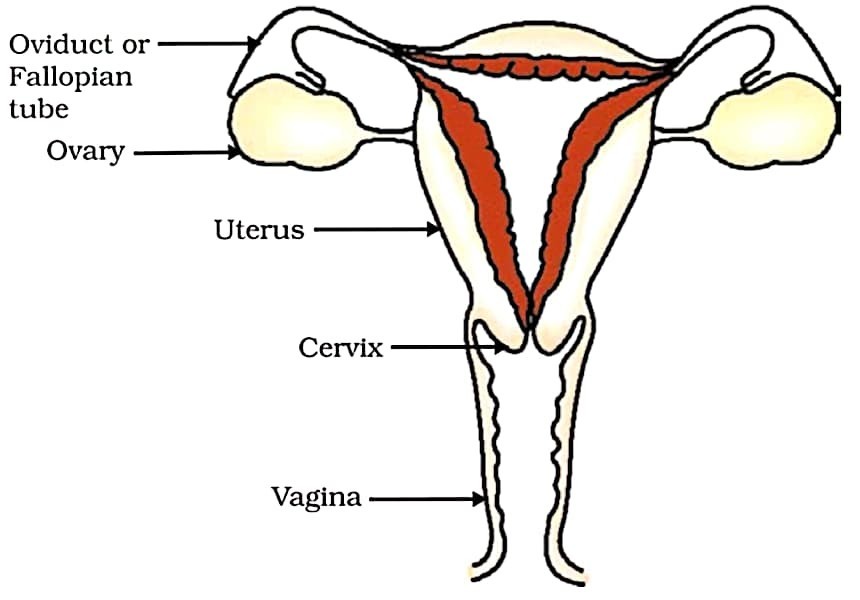
The female germ-cells or eggs are made in the ovaries. They are also responsible for the production of some hormones. On reaching puberty, one egg is produced every month by one of the ovaries. The egg is carried from the ovary to the womb through a thin oviduct or fallopian tube. The two oviducts unite into an elastic bag-like structure known as the uterus.
30. (i) Explain the urine formation in humar beings. (3 Marks)
Answer – Urine is formed in the kidneys through a process known as urine formation or urine production. The process involves three main steps :
• Filtration – Blood from the renal artery enters the glomerulus, a network of tiny blood vessels in the kidney. The pressure in the glomerulus forces water, dissolved substances, and small molecules like urea, uric acid, and creatinine out of the blood and into the Bowman’s capsule, a small sac-like structure surrounding the glomerulus.
• Reabsorption – The filtrate then passes through a series of tubules in the kidney, where most of the water, electrolytes, and nutrients that the body needs are reabsorbed back into the bloodstream. This is an important step to maintain the balance of fluids and electrolytes in the body.
• Secretion – The remaining fluid, which is now urine, is collected in the collecting ducts and transported down to the bladder through the ureters. Along the way, the kidneys can also secrete additional substances into the urine, such as excess potassium or hydrogen ions, to further regulate the body’s fluid balance and pH level.
Once the urine reaches the bladder, it is stored until it is eliminated from the body through the urethra during urination.
(ii) Draw a well labelled diagram of structure of a nephron. (2 Marks)
Answer –
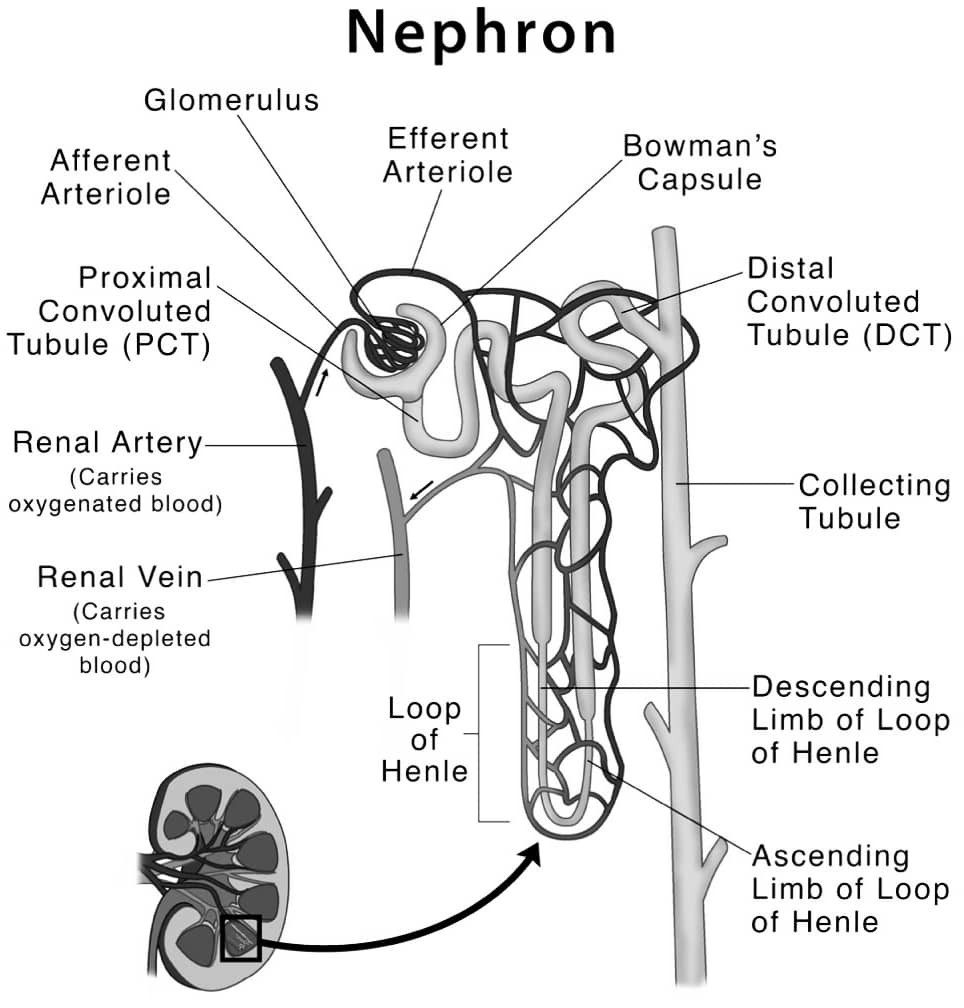
OR
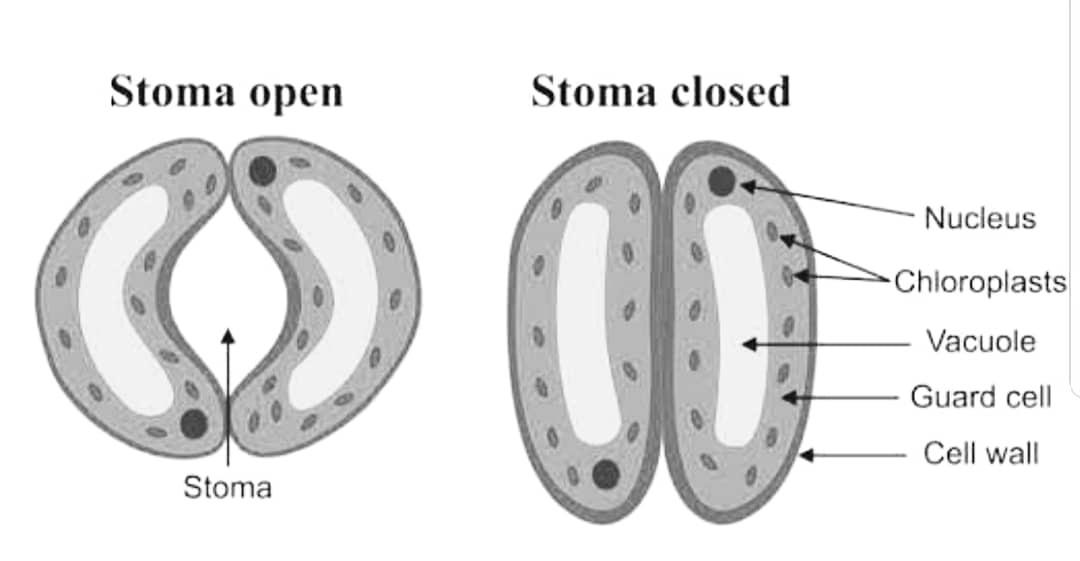
(a) Explain how does the stomatal pore open and close. (2 Marks)
Answer – Stomata are small pores which are present on the surface of the epidermal cells of the plant. It consists of two guard cells, a pore between them and subsidiary cells. The two guard cells have thick walls and are filled with a large amount of cytoplasm.
The guard cells regulate the opening and closing of the stomatal pore. When the guard cells absorb water, they swell up and the stomatal pore opens. This is due to the increase in turgor pressure in the guard cells. On the other hand, when the guard cells lose water, they become flaccid and the stomatal pore closes. This is due to the decrease in turgor pressure in the guard cells.
The opening and closing of the stomatal pore is regulated by the guard cells. The guard cells absorb and lose water depending on the environmental conditions, thus affecting the size of the stomatal pore.
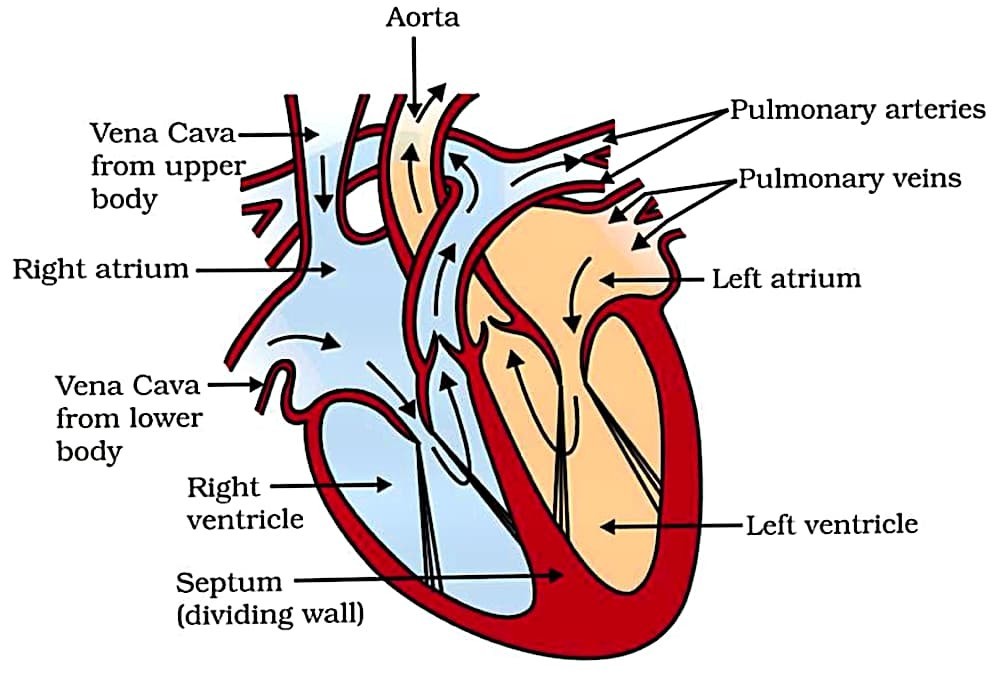
(b) Draw a well labelled diagram of human heart. (3 Marks)
Answer –